Abstract
In this work, gold-incorporated polyethylenedioxythiophene nanocomposite material has been synthesized chemically, employing reverse emulsion polymerization method. Infrared and Raman spectroscopic studies revealed that the polymerization of ethylenedioxythiophene leads to the formation of polymer polyethylenedioxythiophene incorporating gold nanoparticles. Scanning electron microscope studies showed the formation of polymer nanorods of 50–100 nm diameter and the X-ray diffraction analysis clearly indicates the presence of gold nanoparticles of 50 nm in size.
Similar content being viewed by others
Introduction
Conducting polymer (CP) and metal nanoparticle composites or the so-called nanocomposites have received much attention recently due to their potential applications in electrocatalysis, chemical sensors, electrochemical capacitors, and protective coatings against corrosion [1, 2]. Various methods for the preparation of these composites have been described, including electrochemical deposition of nanoparticles onto electrodes previously coated with a CP, photochemical preparation, reduction of metal salts dissolved in a polymer matrix, polymerization of the CP around nanoparticles and mixing of nanoparticles into a polymer matrix. Of the major CPs, polyethylenedioxythiophene (PEDOT) has proven interesting particularly due to its optical transparency in its conducting state, high stability, moderate band gap and low redox potential. Further, it can be polymerized from both organic and aqueous solutions and at both positive and negative potentials, unlike most thiophene derivatives. As PEDOT can be polymerized from aqueous solutions, it could be used in biosensor applications as well.
Au incorporated PEDOT nanomaterials are reported in literature [3–7] employing several techniques, and in this work, we take advantage of the surfactant chemistry to prepare both PEDOT polymer and Au in the nanoform, which ultimately form nanocomposite materials. The present work involves synthesis of PEDOT and Au-incorporated PEDOT nanomaterials through surfactant chemistry and their characterization using Fourier Transform Infrared (FTIR), FT-Raman, Scanning Electron Microscope (SEM) and X-ray diffraction (XRD) techniques.
Materials and Methods
Synthesis of PEDOT and Gold-incorporated Nanoparticles
PEDOT nanoparticles were prepared by reverse cylindrical micelle-mediated interfacial polymerization, according to the method reported elsewhere [8]. Typically, 4.75 g (19.12 mmol) of sodium bis(2-ethylhexyl)sulfosuccinate (AOT) was dissolved in 70 mL of n-hexane, and subsequently 0.36 mL of aqueous FeCl3 solution (10.0 mmol) was introduced in the AOT/hexane reverse cylindrical micelle phase was a yellow viscous solution. Then, 0.25 g of ethylenedioxythiophene (EDOT) solution was added to this solution mixture. After the addition of EDOT monomer there was a slow colour transition from yellow to black, indicating polymerization of the monomer. The polymerization of the EDOT monomer was allowed to proceed for 6 h at 20 °C. Au-incorporated PEDOT nanoparticles were prepared by adding tetrachloroauric acid of (0.25 g) as an oxidant instead of FeCl3 solution in the AOT/hexane solvent mixture. The resultant polymeric substance was washed with acetonitrile/methanol mixture in order to remove AOT and the residual reagents.
XRD measurements were carried out on a Philips Pananalytical X-ray diffractometer using Cu Kα radiation (λ = 0.15406 nm). The identification of the phases was made by referring to the Joint Committee on Powder diffraction Standards International Center for Diffraction Data (JCPDS-ICDD) database. In order to estimate the particle size Scherrer’s equation was used. For this purpose, the (220) peak of the Au fcc structure around 2θ = 64.78° was selected.
SEM measurements were made using Hitachi SEM (Field emission type), model S 4700 with an acceleration voltage of 10 kV. The approximate film composition ( ± 2 at.%) was analysed with an energy-dispersive fluorescence X-ray analysis (XRF-EDX) (Horiba X-ray analytical microscope XGT-2700).
FT-IR spectra were recorded using FT-IR spectrometer (Thermo Nicolet Model 670) equipped with a DTGS detector. All spectra were collected for 256 interferograms at a resolution of 4 cm−1. For Raman spectroscopic measurements, a Thermo-Electron FT-Raman module (InGaAs detector and Nd:YVO4 laser operating at 1064 nm) coupled with a Nexus 670 model FT-IR spectrometer (DTGS detector) was used.
Results and Discussion
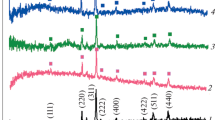
Figure 1 shows the XRD pattern of PEDOT and Au-incorporated PEDOT nanoparticles, prepared by the reverse microemulsion method. As expected for PEDOT, the pattern does not yield any characteristic peaks except the low angle peak at ∼25° indicating the amorphous nature of the polymeric material. The PEDOT–Au nanocomposite shows the diffraction features appearing at 2 theta as 38.20°, 44.41°, 64.54°, 77.50° and 81.68° that correspond to the (111), (200), (220), (311), and (222) planes of the standard cubic phase of Au, respectively. As can be seen, the XRD peaks of the nanocrystallites are considerably broadened compared to those of the bulk gold because of the small size of these crystallites. The average particle size of nanoparticles was estimated based on Scherrer correlation of particle diameter (D) with peak width (As, full width at half maximum, λ = 0.154 nm) for Bragg diffraction from ideal single domain crystallites L = 0.9λ K α1/B (2θ)cos θmax. The average size of the Au particles calculated from the width of the diffraction peak according to the Scherrer equation is ∼50 nm.
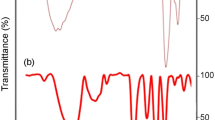
Figure 2 shows the FT-IR spectrum of the PEDOT film together with the monomer spectrum. It is clear that the strong band ascribed to the C–H bending mode at 890 cm−1 disappears in the polymer spectrum in comparison with that of the monomer, demonstrating the formation of PEDOT chains with α,α′-coupling. Vibrations at 1,518, 1,483 and 1,339 cm−1 are attributed to the stretching modes of C=C and C–C in the thiophene ring. The vibration modes of the C–S bond in the thiophene ring can be seen at 978, 842 and 691 cm−1. The bands at 1,213 and 1,093 cm−1 are assigned to the stretching modes of the ethylenedioxy group, and the band around 920 cm−1 is due to the ethylenedioxy ring deformation mode.
The absorption peak at 1,722 cm−1 is usually associated with the doped state of PEDOT. In the case of Au-incorporated polymer matrix, the intensity increases due to the doping of Aunano within the polymer matrix.
Figure 3 shows the Raman spectrum of PEDOT along with the monomer EDOT. In the monomer spectrum, six strong bands dominate the spectrum at 1,487, 1,424, 1,185, 891, 834, and 766 cm−1. In the Raman spectrum of PEDOT, one strong peak at 1,424 cm−1 and a few weaker bands are observed. Also, the other peaks observed in the Raman spectrum of PEDOT are at 1,550 (Quinoid structure), 1,529 (Cα′=Cβ′ stretching), 1,424 (Cα=Cβ stretching), 1,152 (Cα−Cα′ stretching), 986 (Cβ–Calkyl stretching), 851 (C–H bending of 2,3,5-trisubstituted thiophene due to α,α′ polymerization) and 704 cm−1(Cα–S–Cα′ ring deformation). Similar peak patterns were observed for Au-incorporated PEDOT, which indicates that upon incorporation of Au the polymer structure is not affected.
Figure 4 shows the SEM images of the PEDOT nanoform and the Au-incorporated polymer matrix. In general, the morphology of the polymer material shows that the PEDOT nanoparticles formed are uniform in size. The image of the Au-incorporated sample clearly shows discrete areas of high contrast, suggesting the presence of Au. The morphology of the resulting nanocomposites is 50–100 nm in size with incorporated Au nanoclusters. A closer view of the nanoclusters (inset) shows that it comprises of numerous nanoparticles, thus joined to form an aggregate. The formation of gold was also confirmed by EDAX measurements. The oxygen–sulphur of PEDOT and Au nanoparticle ratio is given in Table 1.
From the EDAX measurements, the PEDOT nanoparticle accounts for the presence of oxygen and sulphur within the polymer matrix of 2:1 ratio. Whereas in the case of Au-incorporated polymer matrix, in addition to the oxygen and sulphur peaks it shows the Au peaks which amount to 20 atom wt% in the polymer matrix.
Hence, the above spectral and the surface information indicate that EDOT is polymerized in a linear fashion. The Au nanoparticles are incorporated within the polymer backbone through possible Au–sulphur (thiophene) interactions. The structure of the nanocomposites can be depicted as shown in Scheme 1.
Conclusions
In this work, PEDOT nanoparticles and Au-incorporated PEDOT nanocomposite materials were prepared by reverse cylindrical micellar-mediated interfacial polymerization technique. FT-IR studies clearly reveal the formation of PEDOT upon chemical oxidation of EDOT monomer and the incorporation of gold within the PEDOT matrix. Raman spectral studies revealed that no change occurred in the PEDOT structure upon incorporation of gold. XRD pattern of PEDOT nanoparticle showed the amorphous nature of the material. The diffraction features of the Au-incorporated PEDOT shows standard cubic phase of Au. The broadening of XRD peaks of the nanocrystallites suggests the formation of nanocrystallites and the average size of the gold particles is calculated to be 50 nm. SEM studies of the PEDOT nanoparticle showed that the PEDOT nanoparticles are uniform in size. Discrete areas of high contrast in SEM correspond to gold nanocrystallites of 50–100 nm size.
These nanocomposites when electrochemically prepared using organic media, showed very different morphologies and surface characteristics that enabled their use as selective electrodes in electroanalysis. We are currently pursuing these aspects in the context of sensor applications and will be reported separately.
References
Imisides MD, John R, Wallace GG: Chemtech. 1996, 26: 19.
Gangopadhyay R, De A: Chem. Mater.. 2000, 12: 608. COI number [1:CAS:528:DC%2BD3cXhtlOrtLg%3D] 10.1021/cm990537f
Lee Kim Nam Son Seo M BW JD Y SJ: Mol. Crystallogr. Liq. Crystallogr.. 2003, 407: 1.
Li X, Li Y, Tan Y, Yang C, Li Y: J. Phys. Chem. B. 2004, 108: 5192. COI number [1:CAS:528:DC%2BD2cXislanu78%3D] 10.1021/jp0356618
Senthil Kumar S, Siva Kumar C, Mathiyarasu J, Phani KLN: Langmuir. 2007, 23: 3401. 10.1021/la063150h
Kim BY, Cho MS, Kim YS, Son Y, Lee Y: Synth. Met.. 2005, 153: 149. COI number [1:CAS:528:DC%2BD2MXpvVGkt7k%3D] 10.1016/j.synthmet.2005.07.163
Pacios R, Marcilla R, Pozo-Gonzalo C, Pomposo JA, Grande H, Aizpurua J, Mecerreyes D: J. Nanosci. Nanotechnol.. 2007, 7: 2938. COI number [1:CAS:528:DC%2BD2sXot1enu78%3D] 10.1166/jnn.2007.623
Jang J, Chang M, Yoon H: Adv. Mater.. 2005, 17: 1616. COI number [1:CAS:528:DC%2BD2MXmtlCjur0%3D] 10.1002/adma.200401909
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Selvaganesh, S.V., Mathiyarasu, J., Phani, K. et al. Chemical Synthesis of PEDOT–Au Nanocomposite. Nanoscale Res Lett 2, 546 (2007). https://doi.org/10.1007/s11671-007-9100-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11671-007-9100-6