Abstract
This review introduces quantum dots (QDs) and explores their properties, synthesis, applications, delivery systems in biology, and their toxicity. QDs are one of the first nanotechnologies to be integrated with the biological sciences and are widely anticipated to eventually find application in a number of commercial consumer and clinical products. They exhibit unique luminescence characteristics and electronic properties such as wide and continuous absorption spectra, narrow emission spectra, and high light stability. The application of QDs, as a new technology for biosystems, has been typically studied on mammalian cells. Due to the small structures of QDs, some physical properties such as optical and electron transport characteristics are quite different from those of the bulk materials.
Review
Introduction
In the past years, a new class of fluorescent particles emerged as a good candidate for single molecule and single particle tracking (SPT) in living cells and organisms, the semiconductor quantum dots [1]. Quantum dots (QDs), often described as ‘artificial atoms,’ exhibit discrete energy levels, and their bandgap can be precisely modulated by varying the size [2]. QDs are nanometer-scale semiconductor crystals composed of groups II to VI or III to V elements and are defined as particles with physical dimensions smaller than the exciton Bohr radius [3]. QDs exhibit unique luminescence characteristics and electronic properties such as wide and continuous absorption spectra, narrow emission spectra, and high light stability [4]. They absorb white light and then re-emit a specific color a few nanoseconds later depending on the bandgap of the material [5–7]. QDs are one of the first nanotechnologies to be integrated with the biological sciences [4, 8] and are widely anticipated to eventually find application in a number of commercial consumer and clinical products [9]. For example, CdSe/ZnS quantum dots are presently the most common commercially available product as secondary antibody conjugates that are composed of a core of cadmium selenide ranging from about 10 to 50 atoms in diameter and about 100 to 100,000 atoms in total [10]. QD range is typically between 2 and 10 nm in diameter. QDs consist of a semiconductor core, overcoated by a shell (e.g., ZnS) to improve optical properties, and a cap enabling improved solubility in aqueous buffers [11]. The application of QDs, as a new technology for biosystems, has been mostly studied on mammalian cells. There is an increasing tendency to apply QDs as markers in plant science [12–16]. The application of QDs as markers of the cells or their cell walls for plant bioimaging would be advantageous because of their small size, brightness, independence of emission on the excitation wavelength, and stability under relatively harsh environments. They also have excellent photostability [17] and overcome the limitations associated with photobleaching. Due to the small structures of QDs, some physical properties such as optical and electron transport characteristics are quite different from those of the bulk materials [18]. The study of the impurity states in these low dimensional structures is an important aspect to which many theoretical and experimental works based [16, 19–21]. This review introduces QDs and explores their properties, synthesis, applications, delivery systems in biology, and their toxicity.
Synthesis
Several routes have been used to synthesize QDs [22] but, generally, techniques for QD synthesis used top-down processing methods and bottom-up approach. Top-down processing methods include molecular beam epitaxy (MBE), ion implantation, e-beam lithography, and X-ray lithography. Using the alternative bottom-up approach, colloidal QDs are prepared by self-assemblyin the solution following a chemical reduction [23–26].
In the approaches of top-down, for making the QDs, a bulk semiconductor is thinned. For the achieve QDs of diameter approximately 30 nm, electron beam lithography, reactive-ion etching, and/or wet chemical etching are commonly used. For systematic experiments on quantum confinement effect, controlled shapes and sizes are achievable with the desired packing geometries. Alternatively, focused ion or laser beams have also been used to fabricate arrays of zero-dimension dots. Incorporation of impurities into the QDs and structural imperfections by patterning are major disadvantages with these processes [22].
A number of different self-assembly techniques (bottom-up) have been used to synthesize the QDs, and they may be broadly subdivided into wet-chemical and vapor-phase methods [22]: (a) wet-chemical methods mainly follow the conventional precipitation methods with careful control of parameters for a single solution or mixture of solutions. The precipitation process invariably involves both nucleation and limited growth of nanoparticles. Nucleation may be categorized as homogeneous, heterogeneous, or secondary nucleation [27]. Homogeneous nucleation occurs when solute atoms or molecules combine and reach a critical size without the assistance of a pre-existing solid interface. Wet-chemical methods are generally microemulsion, sol–gel [28–30], competitive reaction chemistry, hot-solution decomposition [31–33], sonic waves or microwaves [34], and electrochemistry. (b) Vapor-phase methods for producing QDs begin with processes in which layers are grown in an atom-by-atom process. Consequently, self-assembly of QDs occurs on a substrate without any patterning [35–38]. Self-assembly of nanostructures in material grown by MBE, sputtering, liquid metal ion sources, or aggregation of gaseous monomers are generally categorized under vapor-phase methods [22]. MBE has been mainly used to self-assemble QDs from III-V semiconductors and II-VI semiconductors using the large lattice mismatch, e.g., InAs on GaAs has a 7% mismatch and leads to SK growth [35].
Applications
In this review, we evaluate few experiments that show the high potential of QDs in biological application, including tracking different macromolecules in the cell, tracking various cells in the tissue, labeling organelles and cells, clinical applications, and other applications [39–43].
QDs for labeling cells
Because QDs have constant and unique optical properties, they are the best candidate for cell labeling, as compared with organic dyes.
Use in plant bioimaging There is an increasing application of QD as markers for the cells or cell walls (CWs) in plant science. A first target location for external agents in a plant cell is the CW [44]. Djikanović et al. demonstrated that CdSe QDs bind typically to cellulose and lignin in the cell wall of Picea omorika branch. Respectively, binding to lignin and cellulose are achieved by interaction with the chains of C = C and C-C alternating bonds and interaction with the OH groups [44]. Data showed that QDs are suitable for homogenous marking of the whole cell wall. This is a consequence of the structural arrangement of the cell wall polymers in the whole cell wall network as well as the extremely small size of the QDs. These characteristics enable a feasible penetration of the nanoparticles inside the polymer structures in the CW composite [44].
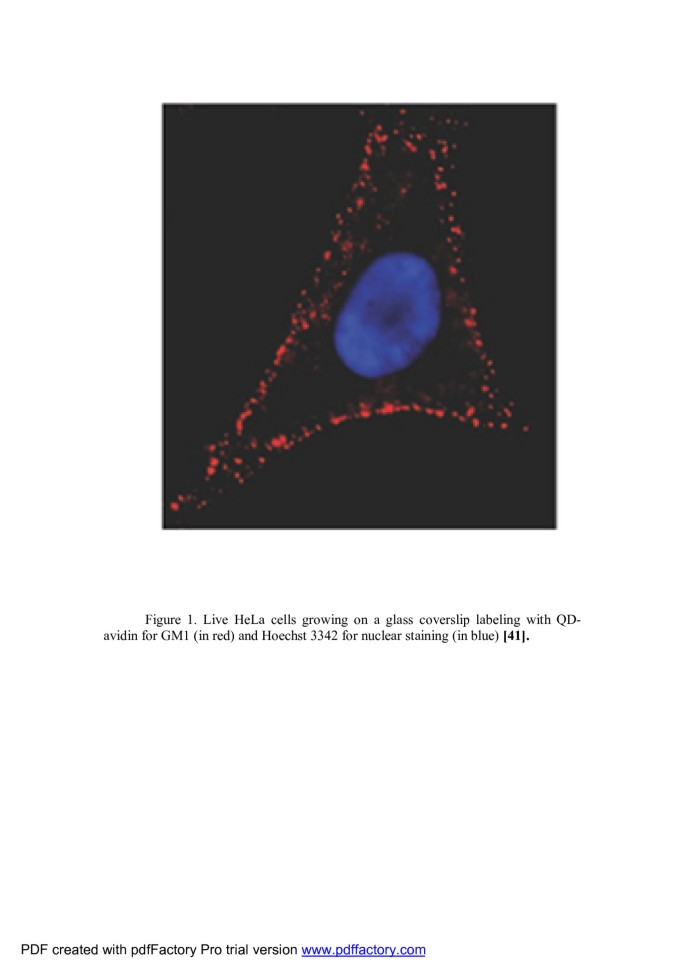
Use in animal bioimaging Goldman et al. used biotinylated CTxB in conjunction with QD-avidin conjugates [45] for labeling of the live HeLa cells which Figure 1 shows an image of the lateral membrane staining for GM1 ganglioside using QDs (in red) and nuclear staining using Hoechst (in blue). Punctuate labeling of the cell surface by QD bioconjugate is typical for molecules such as GM1 that is present in membrane rafts [46].
Live HeLa cells growing on a glass coverslip. Labeled with QD-avidin for GM1 (in red) and Hoechst 3342 for nuclear staining (in blue) [46].
In another study, they labeled live HeLa cells which were biotinylated using sulfo-NHS-SS biotinylating reagent and then incubated with the avidin-conjugated yellow-emitting QDs. It is shown in Figure 2[47].
HeLa cells labeled with the avidin-conjugated yellow-emitting QDs.[47]. (A) Image of cells immediately after the unbound QDs were removed in which labeling is restricted to the cell surface. (B) Image of a cell that was allowed to grow for 2 h after washing out of unbound QDs.
For long-term live cell imaging, Hasegawa et al. used the CHPNH2-QD complexes which were uniformly internalized into the cells without being aggregated. Therefore, CHPNH2 nanogel has high potential for use in long-term live cell imaging. The interaction of QDs with cells was successfully controlled by the amino group content of the CHPNH2 nanogel [48].
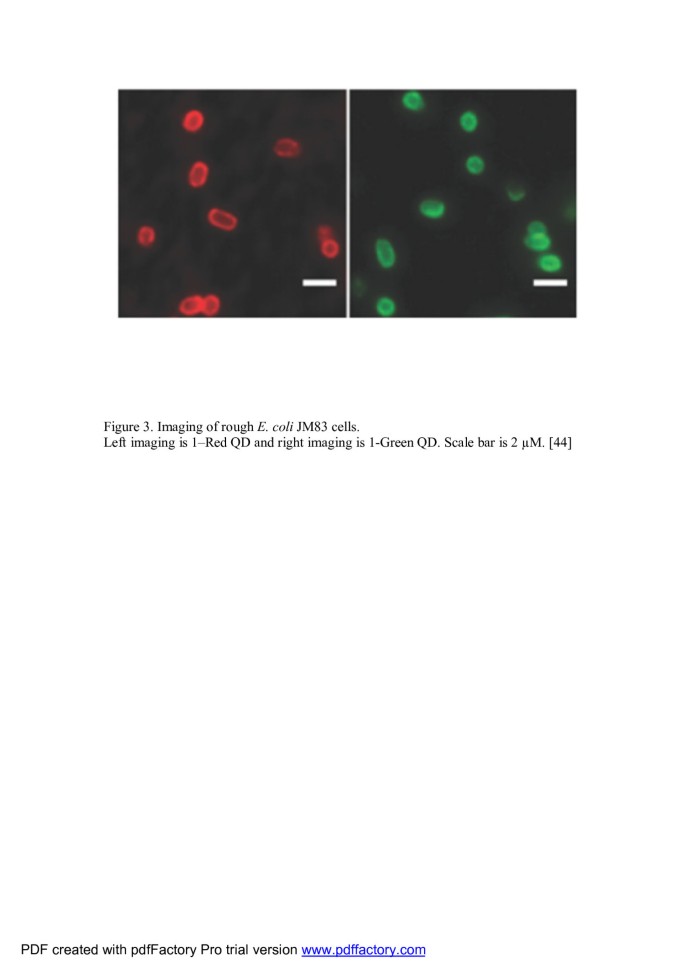
Use in prokaryote bioimaging Sensitive and selective staining of bacterial mutants using QD labels was demonstrated by Smith's group. This principle of detection is based on selective targeting affinity of Zn(II)-dipicolylamine coordination complex to phospholipids on the bacterial cell surface of specific strain as shown in Figure 3[49, 50].
Imaging of rough Escherichia coli JM83 cells. Left imaging is red QD, and right imaging is green QD. Scale bar is 2 μM [49].
In another study, authors demonstrated the use of magnetic beads coated with anti-E.coli O157 antibodies and streptavidin-coated QDs for measuring the bacterial cell concentration [51]. Yang and Li, using QDs with different emission wavelengths (525 nm and 705 nm), reported the simultaneous detection of E. coli O157:H7 and Salmonella typhimurium[52].
Tracking different particles
With the application of new imaging methods and the use of brighter and more stable probes, such as QDs, single particle tracking has the potential to enter into a new era of high resolution and long timescale imaging [53–55]. SPT techniques allow scientists to follow single molecules in real time and visualize the actual molecular dynamics in their habitant environment.
For extracellular study Because QDs do not require intracellular delivery through the impermeable plasma membrane, membrane receptors or membrane-associated proteins are intuitive targets for QD imaging [53]. Howarth et al. demonstrated a method to track endogenous cell-surface proteins without cross-linking by purifying monovalent antibody-QD conjugates. They approach to make monovalent tight-binding QDs, using mSA, which could be applied to other nanoparticles that show sufficient electrophoretic mobility. They applied sQD-mSA1 to study the mobility of a mutant of low-density lipoprotein (LDL) receptor with a truncated cytosolic tail, originally found from an individual with familial hypercholesterolemia. This mutant phenotype has been extensively investigated by following LDL, but Howarth and co-workers analyzed the behavior of the receptor itself (supplementary methods). They imaged single monovalent sQDs bound to the biotinylated AP-LDL receptor, as indicated by QD fluorescence intensity and blinking. The mobility of mutant receptors labeled with sQD-mSA1 was significantly greater than that of labeled wild-type LDL receptor (P = 1.6 × 10−14) [56].
In similar studies, recently, QDs used to target membrane proteins and investigate the mobility and entry-exit kinetics in several systems: (1) various transmembrane proteins, for example, integrins [57], channels [58], and aquaporines [59]; (2) receptors GABA [60], glycine [61], interferon [62], and HER [63, 64]; and (3) neurological synapse [65, 66].
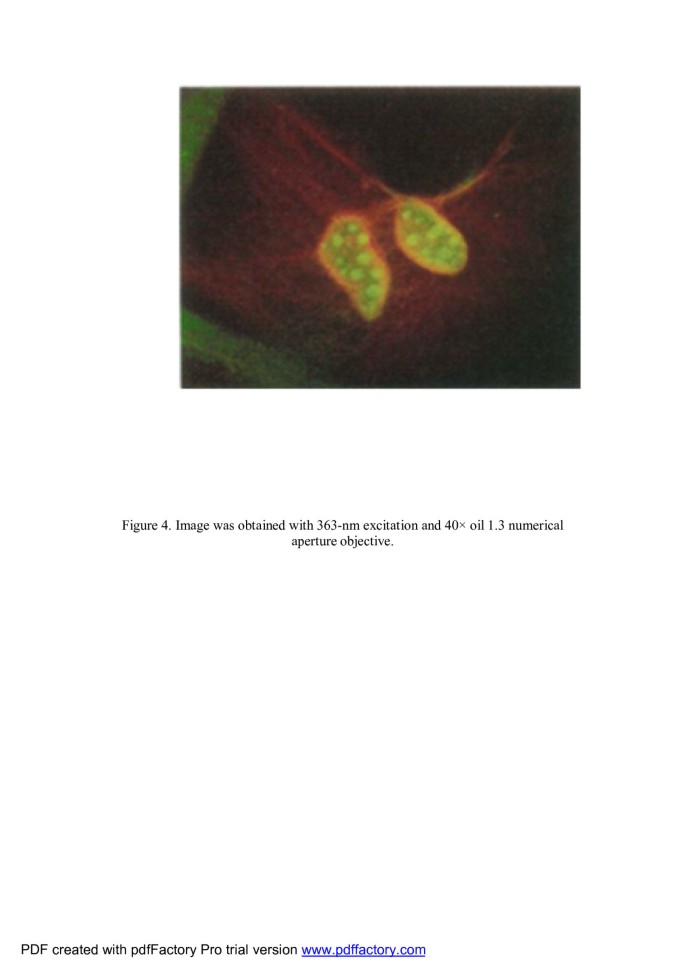
For intracellular study In one of the study, the advantages of the broad, continuous excitation spectrum were demonstrated in a dual-emission, single-excitation labeling experiment on mouse fibroblasts. These nanocrystal probes are, thus, complementary and, in some cases, may be superior to existing fluorophores [4]. Nonspecific labeling of the nucleus by both the red and the green probes resulted in a yellow color. The red actin filaments were specifically stained. Also, the green probes penetrate into the nucleus. Both are shown in Figure 4[4].
This is shown as green color for nucleus and red color for actin filaments. Nonspecific labeling of the nucleus by both the red and the green probes resulted in a yellow color [4].
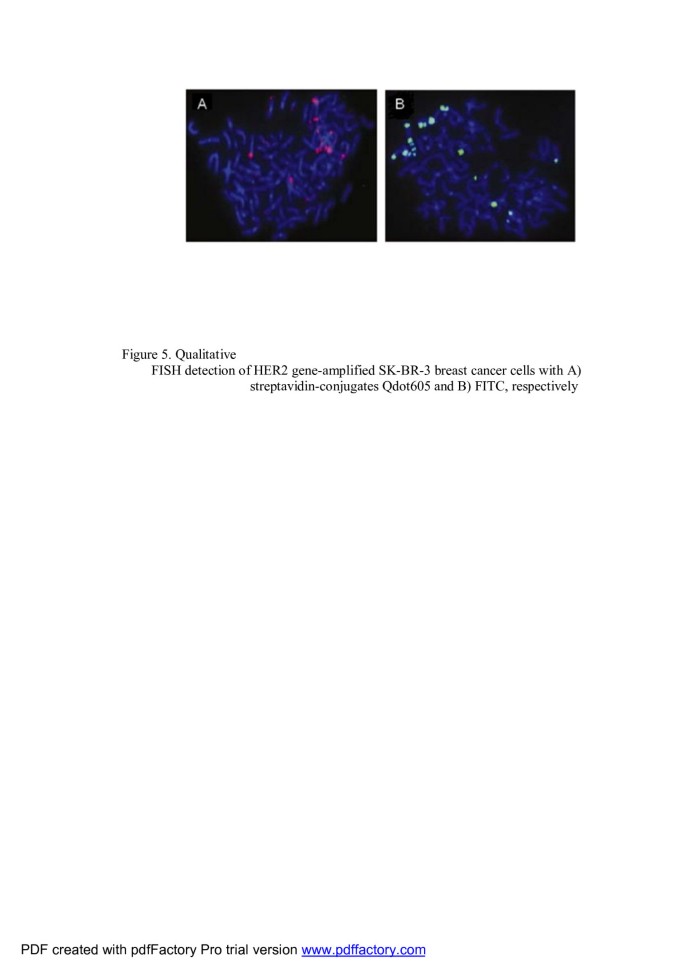
Superior stability of QD fluorophores gives the possibility to improve quantitation of FISH analysis of human chromosomal changes. Xiao and Barker have investigated coated (CdSe)ZnS QDs as fluorescence labels for FISH of biotinylated DNA to human lymphocyte metaphase chromosomes under conditions that approximate those commonly found in clinical cytogenetics laboratories [67]. They have also demonstrated the application of QDs to FISH detection of the clinically relevant HER2 locus in breast cancer cells (Figure 5).
Qualitative FISH detection of HER2 gene-amplified SK-BR-3 breast cancer cells. With (A) streptavidin-conjugated Qdot605 and (B) FITC, respectively [67].
Pierobon et al. [68] and Nelson et al. [69] tagged myosin V molecules with QDS toestablish a link between in vitro and in-cell measurements of myosin V motors. Then, the complex myosin V/QD (MyoV::QD), using the pinocytic influx, was introduced into the cells.
Yoo et al. [70] and Courty et al. [71] characterized the dynamics of other major actors of intracellular transport: the kinesin-1, the actin filaments, and the microtubules [65].
Imaging in situ
Imaging of the satellite cells in rat intact and injured soleus muscles using quantum dots The employment of satellite cells, which are located between the basement membrane and the plasma membrane in myofibers, is required for myofiber repair after muscle injury or disease. Using QDs conjugated to anti-M-cadherin antibody, Ishido and Kasuga attempted the visualization of satellite cells in both intact and injured skeletal muscle of rat in situ. They demonstrated in situ real-time imaging of satellite cells localized within the skeletal muscle (Figure 6) [72].
Double fluorescence staining to visualize the localization of M-cadherin (in red) and nuclei (in blue). Arrows indicate that M-cadherin-positive satellite cells were located within the intact soleus muscle in situ[72].
Imaging morphogenesis in Xenopus with quantum dot nanocrystals Stylianou and Skourides are the first to report the use of near-infrared QDs to image mesoderm migration in vivo with single cell resolution and provide quantitative in vivo data regarding migration rates [73].
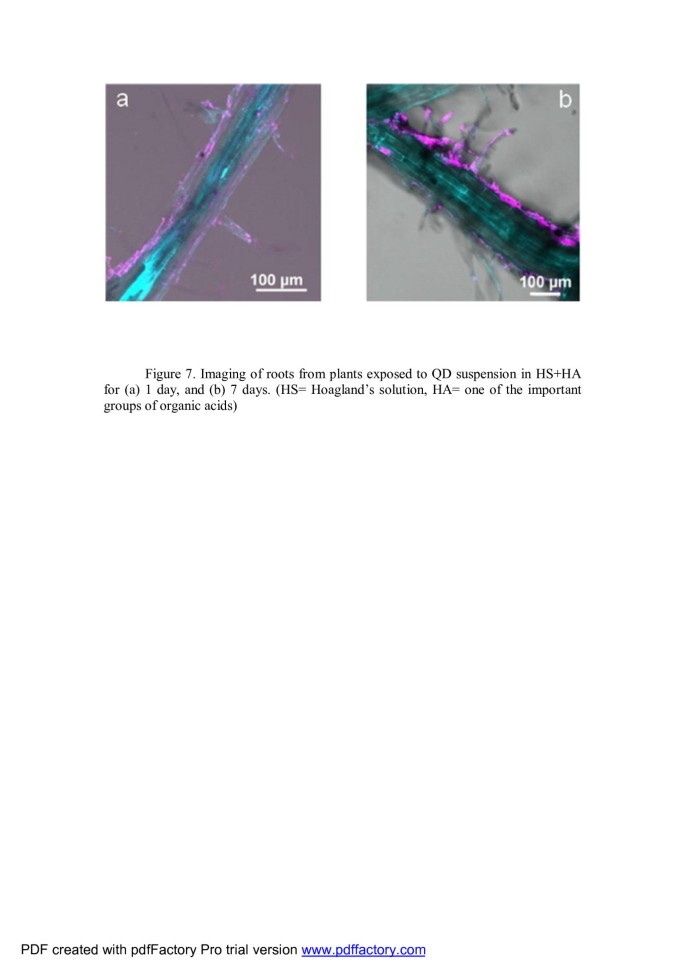
Navarro et al. experiments revealed that Arabidopsis exposed to QDs that are dispersed in Hoagland's solution for 1 to 7 days did not internalize intact QDs. Fluorescence microscopy showed strong evidence that the QDs were generally on the outside surfaces of the roots (Figure 7). The amount of QDs adsorbed is dependent on the stability of the QDs in suspension [74].
Imaging of roots from plants exposed to QD suspension in HS + HA. For (A) 1 and (B) 7 days [74].HS, Hoagland's solution; HA, one of the important groups of organic acids.
Using QDs in clinical applications
The development of multifunctional nanomaterials combining diagnostic and therapeutic purpose has recently attracted intensive interests [75–81]. In this paper, we have reviewed the clinical applications of QDs in the three categories that include: (1) biomarker detection in various cancers, (2) imaging and sensing of infectious diseases, and (3) other clinical therapeutic applications.
Biomarker detection in various cancers using QDs The detection of cancer biomarkers is important for diagnosis, disease stage forecasting, and clinical management [82]. QDs with intense and stable fluorescent properties could enable the detection of tens to hundreds of cancer biomarkers in blood assays, on cancer tissue biopsies, or as contrast agents for medical imaging. Clinical outcome of cancer diagnosis is highly dependent on the stage at which the malignancy is detected, and therefore, early screening has become extremely important in any type of cancer [83].
-
1.
Multicolor and multiplexing potentialities of QDs are used for the detection of four protein biomarkers CD15, CD30, CD45, and Pax5 of Hodgkin's lymphoma from lymphoma tissues. Simultaneous visualization using multiplexed QD staining was advantageous for the selective identification of rare Hodgkin (Reed-Sternberg) cells, a primary diagnostic target for Hodgkin's disease, which was not achievable using traditional immunohistochemistry assays [84, 85].
-
2.
Yu et al. reported the use of GSH-TGA-QDs-ND-1 probes to label colorectal cancer cells CCL187. They prepared QDs, which were conjugated with monoclonal antibody ND-1 for specific reaction with antigen LEA [86].
-
3.
In the United States, pancreatic cancer is the fourth leading cause of cancer death (about 18,770 men and 18,030 women (36,800 people) in 2010) [87]. Using semiconductor QD-antibody conjugates, Lee et al. demonstrated quantitative profiling of biomarkers for pancreatic cancer at the single-cell level. Their results show the possibility of this method for staging and forecasting, such as prostate stem cell antigen claudin-4, and mesothelin, which are expressed in different stages of progression of pancreatic cancer [82]. Anyway, realizing quantitative profiling requires stable quantum yield, monodisperse QD-Ab conjugates, and well-defined surface chemistry [88].
There are evidences showing the application of QDs in micro- and nanoarrays for the detection of cancer biomarkers [83].
Imaging and sensing of infectious diseases by QDs QDs have become one of the most hopeful and interesting materials for diagnostic applications of bioimaging, labeling, and sensing for infectious diseases such as respiratory syncytial virus (RSV)that isone of the families of Paramyxoviridae [50]. In Table 1, some of the infectious diseases and QDs used to distinguish them are shown.
-
1.
QDs for assessing axon growth
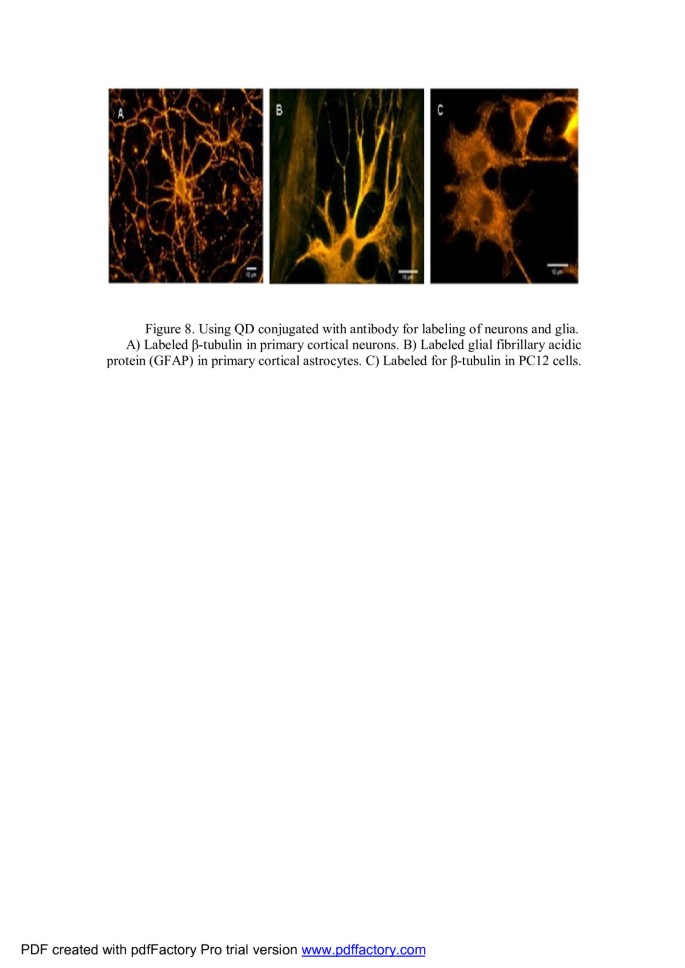
A major health problem with injuries to the spinal cord and brain is traumatic central nervous system injury reporting of approximately 265,000 and 1.5 million new injuries each year [103–105]. QDs represent a new device of significant potential in neuroscience research, and they are useful for experiments that are limited by the restricted anatomy of neuronal and glial interactions [106]. One of the problems in treatment is estimating its effectiveness. They allow the ability to visualize and track dynamic molecular processes over long times (Figure 8) [106]. Application of surface-engineered QDs is an area of nanotechnology probing the details of cellular and molecular processes in neuronal cells [4, 107–109]. QD bioconjugates based on surface chemistry can be broadly classified as follows: (1) QDs' surface modified by bioactive molecules and (2) QD-polymer nanocomposites [103]. This advance might be significantly important to assess axon growth pending the regeneration process [103]. Previous investigations were demonstrated in Table 2.
-
2.
QD used as a probe in an anti-malarial drug-screening assay
Malaria is a major global health problem, threatening over 300 million people and causing nearly one million deaths annually [114, 115]. Tokumasu et al. used QD-Ab to demonstrate the distinct pattern of distribution of protein and to observe erythrocyte membrane deformation occurring duringthe invasion of erythrocytes by Plasmodium falciparum[116]. Ku et al. showed a simple and efficient method to label P. falciparum-infected RBC using a QD-based probe and its applicability as an efficient probe for anti-malarial drug screening [115].
Using QD conjugated with antibody for labeling of neurons and glia. (A) Labeled β-tubulin in primary cortical neurons. (B) Labeled glial fibrillary acidic protein in primary cortical astrocytes. (C) Labeled for β-tubulin in PC12 cells [106].
Other applications
QDs as pH probes for the study of enzyme reaction kinetics[117] Lately, worth advancement has been achieved in water-soluble QDs as ionic probe. Jin et al. reported the use of modified CdSe QDs for the sensitive determination of cyanide ions [C ≡ N]−[117, 118]. Xie et al. reported the determination of Cu2+ by using CdSe/ZnS QDs modified with bovine serum albumin [119]. QDs also have been reported to be sensitive to pH [120–125]. The sensitivity of QDs' photoluminescence to pH, improve stability, and a monitoring range for the determination of proton concentration, which is maybe due to a function of surface modifications and effects on exciton trap sites, leads to applications utilizing QDs as pH probes [126]. Water-soluble QDs, ZnS, modified with mercaptoacetic acid (MAA) were sensitive to environmental factors and found to be a satisfactory pH probes that could have potential applications in chemical and biochemical sensing. Using the modified QD surface, they were applied as pH probes in monitoring the hydrolysis of glycidyl butyrate which is catalyzed by porcine pancreatic lipase (PPL) [117].
QDs use for protein micro- and nanoarrays to the detection of cancer biomarkers Protein microarrays are useful device as highthroughput screening tools in proteomics [127–129], for biosensing purpose [130], new drug discovery [131], and enabling a quick parallel screening method for the detection of protein-protein interactions in case of large protein populations. There are various reports in which QDs have been used in microarray fabrication such as sandwich-based immunoassay type, RP protein microarray type, etc. [132–135]. Here, IgG detection was done on a glass chip using a QD-labeled secondary Abs as sandwich assay approach. In RP protein microarrays, Geho et al. used pegylated QDs conjugated with streptavidin as detection elements. In another study, Zajac et al. investigated the ability of the platform to detect different cytokines TNF-α, IL-8, IL-6, MIP-1β, IL-13, and IL-1β using two different models of quantum dot probes. Their results demonstrated high sensitivity of the investigated detection system with less than picomolar concentration [136]. Kerman et al. reported the use of QDs for detection cell lysates spiked with DNA-PK proteins with the help of mAb, in an RP protein microarray format. Kerman et al. make immunosensor based on QD for the detection of prostate specific antigen (PSA) in a sandwich assay approach for chip fabrication [134]. Gokarna et al. used pegylated QD-conjugated PSA Abs to demonstrate the fabrication of a cancer protein biochip for the detection of PSA, which is a biomarker for prostate cancer. The QD nonspecificity can show to be quite detrimental to some extent in case of multiplexed assay systems where multiple proteins are to be detected simultaneously [83].
QD delivery Due to the unique properties of QDs, they are best tools for intracellular studies such as visualizing the cellular structure, studying the dynamic cellular processes, and tracking single molecules in the cell [137, 138]. To achieve this goal, translocation of functionalized QDs into the cell for labeling organelles and tracking single molecules is important. QDs have hydrophobic surface and have a little toxicity, therefore cannot be applied in vivo unless their surface is modified. Thus, by surface modification, their hydrophilicity will increase but their toxicity will decrease.
Hasegawa et al. used nanogel-QD hybrid nanoparticles for live cell imaging [48]. They also confirmed the cellular uptake of CHPNH2(15)-QD nanoparticles using other normal cells (TIG-3 and MRC-5) and cancer cells (T24, Saos-2, T98G, A549, MCF-7, and YKG-1) (Figure 9) [48].
Confocal laser scanning fluorescence microscopyimages of cells labeled with CHPNH 2 (15)-QD nanoparticle. (A) TIG-3 cells, (B) MRC-5 cells, (C) MCF-7 cells, and (D) YKG-1 cells [48].
In recent years, functional peptides that transmit biomaterials into cells have been developed in biomaterial research. Because of lysosomal trapping, QD delivery into cells with conjugated cell-penetrating peptides by the endocytic pathway was challenging in biomedical applications [139]. In another study, engineered peptides for producing QDs tagging protein ligands and biosensors to their surfaces, by appropriate cysteines or histidines, have served as ligands [140]. Encapsulation of QDs in viral capsids provides a new tool which allows the design of intracellular microscopic probes and vectors [141]. More samples of QD delivery systems are shown in Table 3.
Toxicity of QDs
There are different opinions about the toxicity of QDs; therefore, we investigated their toxicity in amoeba as primary eukaryotes, in plant, and in animal.
In amoeba
It has been determined that QD labeling had no detectable effect on cell growth and had no deleterious effects on cellular signaling and motility during development of the Dictyostelium discoideum cells [47].
In plant
The ratio of reduced glutathione levels (GSH) relative to the oxidized glutathione (GSSG) in plants suggests that QDs caused oxidative stress on the plant at this condition [74].
In animal
Yan et al. investigated the potential vascular endothelial toxicity of mercaptosuccinic acid (2-sulfanylbutanedioic acid)-capped QDs in vitro. Their results suggested that QDs could not only impair mitochondria but also exert endothelial toxicity through activation of mitochondrial death pathway and induction of endothelial apoptosis [156].
More recently, Chen et al. have studied the cytotoxicity of CdTe/CdS (core-shell) structured and also CdTe/CdS/ZnS (core-shell-shell) structured aqueous synthesized QDs, and their results suggest that the cytotoxicity of CdTe QDs not only comes from the release of Cd2+ ions but also intracellular distribution of QDs in cells and the associated nanoscale effects [157]. Table 4 demonstrated more results for toxicity of QDs [158–162].
Conclusions
In this review, we summarize few experiments that illustrate the high potential of QDs used for/as:
-
1.
labeling biomolecules and cells;
-
2.
tracer to follow the intracellular/extracellular dynamic of a single biomolecule/cell;
-
3.
localization of biomolecules in vitro/in vivo;
-
4.
imaging of biomolecules or cells in vitro/in vivo;
-
5.
assessing cell growth in damaged tissue;
-
6.
pH probes for the study of enzyme reaction kinetics;
-
7.
biomarker detection in various cancers;
-
8.
imaging and sensing of infectious diseases; and
-
9.
protein micro- and nanoarrays to the detection of cancer biomarkers.
These studies have been generated using QDs because of their small size, brightness, independence of emission on the excitation wavelength, and stability under relatively harsh environments which would be advantageous. In contrast, there are different opinions about the toxicity and fate of QDs in vivo. Therefore, more experiments should be done, and much more data should be available, to be sure to do clinical trials on humans.
Future prospects
In the future, QDs will be used for identifying various categories of cancer cells, the molecular mechanisms of disease, and new drug action mechanisms, applying them in the intracellular/extracellular studies, and making new methods for biochemical assaying.
References
Pierobon P, Cappello G: Quantum dots to tail single bio-molecules inside living cells. Adv Drug Deliv Rev 2012, 64(2):167–178. 10.1016/j.addr.2011.06.004
Klimov VI: Spectral and dynamical properties of multiexcitons in semiconductor nanocrystals. Annu Rev Phys Chem 2007, 58: 635–673. 10.1146/annurev.physchem.58.032806.104537
Chan WC, Maxwell DJ, Gao X, Bailey RE, Han M: Luminescent quantum dots for multiplexed biological detection and imaging. Curr Opin Biotechnol 2002, 13(1):40–46. 10.1016/S0958-1669(02)00282-3
Bruchez M, Moronne M, Gin P, Weiss S, Alivisatos AP: Semiconductor nanocrystals as fluorescent biological labels. Science 1998, 281(5385):2013–2016.
Deb P, Bhattacharyya A, Ghosh SK, Ray R, Lahiri A: Excellent biocompatibility of semiconductor quantum dots encased in multifunctional poly(N-isopropylacrylamide) nanoreservoirs and nuclear specific labeling of growing neurons. Appl Phys Lett 2011, 98(10):103702–103703. 10.1063/1.3562036
Dabbousi BO, Rodriguez-Viejo J, Mikulec FV, Heine JR, Mattoussi H, Ober R: (CdSe)ZnScore − shell quantum dots: synthesis and characterization of a size series of highly luminescent nanocrystallites. J Phys Chem B 1997, 101(46):9463–9475. 10.1021/jp971091y
Bakalova R, Ohba H, Zhelev Z: Quantum dots as photosensitizers? Nat Biotechnol 2004, 22(11):1360–1361. 10.1038/nbt1104-1360
Chan WC, Nie S: Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science 1998, 281(5385):2016–2018.
Azzazy HM, Mansour MM, Kazmierczak SC: From diagnostics to therapy: prospects of quantum dots. Clin Biochem 2007, 40(13–14):917–927.
Deerinck TJ: The application of fluorescent quantum dots to confocal, multiphoton, and electron microscopic imaging. Toxicol Pathol 2008, 36(1):112–116. 10.1177/0192623307310950
Ghasemi Y, Peymani P, Afifi S: Quantum dot: magic nanoparticle for imaging, detection and targeting. Acta Biomed 2009, 80(2):156–165.
Corredor E, Testillano PS, Coronado MJ, González-Melendi P, Fernández-Pacheco R, Marquina C: Nanoparticle penetration and transport in living pumpkin plants: in situ subcellular identification. BMC Plant Biol 2009, 9: 45. 10.1186/1471-2229-9-45
Lin S, Meyer DE, Curran MA: Uptake, translocation, and transmission of carbon nanomaterials in rice plants. Small 2009, 5(10):1128–1132.
Muller F, Houben A, Barker PE, Xiao Y, Käs JA, Melzer M: Quantum dots – a versatile tool in plant science? J Nanobiotechnology 2006, 4: 5. 10.1186/1477-3155-4-5
Santos AR, Miguel AS, Tomaz L, Rui Malhó , Christopher : The impact of CdSe/ZnS quantum dots in cells of Medicago sativa in suspension culture. J Nanobiotechnology 2010, 8: 24. 10.1186/1477-3155-8-24
Wu YL, Lim CS, Fu S, Tok AIY, Lau HM, Boey FYC: Surface modifications of ZnO quantum dots for bio-imaging. Nanotechnology 2007, 18(21):215604. 10.1088/0957-4484/18/21/215604
Wu YL, Lim CS, Fu S, Tok AIY, Lau HM, Boey FYC: Water-soluble quantum dots for biomedical applications. Biochem Biophys Res Commun 2006, 348(3):781–786. 10.1016/j.bbrc.2006.07.160
Arnot HEG, Watt M, Sotomayor-Torres CM, Glew R, Cusco R, Bates J, Beaumont SP: Photoluminescence of overgrown GaAs-GaAlAs quantum dots. Superlattices and Microstructures 1989, 5(3):459–463. 10.1016/0749-6036(89)90333-9
Li SS, Xia JB: Electronic structure and binding energy of a hydrogenic impurity in a hierarchically self-assembled GaAs/AlxGa1 - xAs quantum dot. J Appl Phys 2006, 100(8):083714. 10.1063/1.2358406
Li S-S, Xia J-B: Electronic states of a hydrogenic donor impurity in semiconductor nano-structures. Physics Letters A 2007, 366(1–2):120–123.
Li SS, Kong XJ: Hydrogenic impurities in GaAs-Ga1-xAlxAs superlattices in an axial magnetic field. J Phys Condens Matter 1992, 4(20):4815. 10.1088/0953-8984/4/20/008
Bera D, Qian L, Tseng T-K, Holloway PH: Quantum dots and their multimodal applications: a review. Materials 2010, 3(4):2260–2345. 10.3390/ma3042260
Mattoussi H, Palui G, Na HB: Luminescent quantum dots as platforms for probing in vitro and in vivo biological processes. Adv Drug Deliv Rev 2012, 64(2):138–166. 10.1016/j.addr.2011.09.011
Birudavolu S, Nuntawong N, Balakrishnan G, Xin YC, Huang S, Lee SC, Brueck SRJ, CP : Selective area growth of InAs quantum dots formed on a patterned GaAs substrate. Appl Phys Lett 2004, 85(12):2337–2339. 10.1063/1.1792792
Nakata Y, Mori T, Seki H: Molecular beam epitaxial growth of InAs self-assembled quantum dots with light-emission at 1.3 μm. Journal of Crystal Growth 2000, 208(1–4):93–99.
Yamilov A, Herrera MR, Bertino MF: Quantum dots by ultraviolet and x-ray lithography. Nanotechnology 2007, 18(31):315603. 10.1088/0957-4484/18/31/315603
Burda C, Chen X, Narayanan R, El-Sayed MA: Chemistry and properties of nanocrystals of different shapes. Chem Rev 2005, 105(4):1025–1102. 10.1021/cr030063a
Bang J, Fau Yang H, Holloway PH: Enhanced and stable green emission of ZnO nanoparticles by surface segregation of Mg. Nanotechnology 2006, 17(14):973.
Spanhel L, Anderson MA: Semiconductor clusters in the sol–gel process: quantized aggregation, gelation, and crystal growth in concentrated zinc oxide colloids. J Am Chem Soc 1991, 113(8):2826–2833. 10.1021/ja00008a004
Bera D, Qian L, Sabui S, Santra S: Photoluminescence of ZnO quantum dots produced by a sol–gel process. Opt Mater 2008, 30(8):1233–1239. 10.1016/j.optmat.2007.06.001
Qu L, Peng X: Control of photoluminescence properties of CdSe nanocrystals in growth. J Am Chem Soc 2002, 124(9):2049–2055. 10.1021/ja017002j
Murray CB, Norris DJ, Bawendi MG: Synthesis and characterization of nearly monodisperse CdE (E = sulfur, selenium, tellurium) semiconductor nanocrystallites. J Am Chem Soc 1993, 115(19):8706–8715. 10.1021/ja00072a025
Qu L, Peng ZA, Peng X: Alternative routes toward high quality CdSe nanocrystals. Nano Lett 2001, 1(6):333–337. 10.1021/nl0155532
Li L, Qian H, Ren J: Rapid synthesis of highly luminescent CdTe nanocrystals in the aqueous phase by microwave irradiation with controllable temperature. Chem Commun (Camb) 2005. 10.1039/B412686F
Xin SH, Yin A, Kim C, Dobrowolska M, Merz JL: Formation of self-assembling CdSe quantum dots on ZnSe by molecular beam epitaxy. Appl Phys Lett 1996, 69(25):3884–3886. 10.1063/1.117558
Leonardi K, Selke H, Heinke H, Ohkawa K, Hommel D, Gindele F, Woggon U: Formation of self-assembling II–VI semiconductor nanostructures during migration enhanced epitaxy. J Crystal Growth 1998, 184–85: 259–263.
Kurtz E, Shen J, Schmidt M, Grun M, Hong SK, Litvinov D: Formation and properties of self-organized II–VI quantum islands. Thin Solid Films 2000, 367(1–2):68–74.
Swihart MT: Vapor-phase synthesis of nanoparticles. Curr Opin Colloid Interface Sci 2003, 8(1):127–133. 10.1016/S1359-0294(03)00007-4
Zhang ZY, Oehler AEH, Resan B, Kurmulis S, Zhou KJ, Wang Q, Mangold M, Süedmeyer T, Keller U, Weingarten KJ, Hogg RA: 1.55 μm InAs/GaAs quantum dots and high repetition rate quantum dot SESAM mode-locked laser. Nano Lett 2010, 10: 1512–1516. 10.1021/nl100217k
Yang H, Luan W, Shan-tung T, Wang ZM: Synthesis of nanocrystals via microreaction with temperature gradient: towards separation of nucleation and growth. Lab Chip 2008, 8: 451–455. 10.1039/b715540a
Jiang W, Wang ZM, Dorogan VG, Mazur YI, Shibin L: Gregory, Insight into optical properties of strain-free quantum dot pairs. Journal of Nanoparticle Research 2011, 13: 947–952. 10.1007/s11051-010-0219-5
Yuechao J, Xiaoyong G, Jingxiao L, Yongsheng C, Jianpeng Z, Xinli L: A novel method for PbS quantum dot synthesis. Mater Lett 2012, 72: 116–118.
Zhiming M, Wang D, Liang B, Sablon KA, Jihoon L, Yuriy I, Mazur D, Strom NW, Gregory J, Salamo D: Self-organization of InAs quantum-dot clusters directed by droplet homoepitaxy. Small 2007, 3: 235–238. 10.1002/smll.200600330
Kalauzi A, Mutavdžić D, Djikanović D, Radotić K, Jeremić M: Interaction of the CdSe quantum dots with plant cell walls. Colloids Surf B Biointerfaces 2012, 91: 41–47.
Goldman ER, Balighian ED, Mattoussi H, Kuno MK, Mauro JM, Tran PT, Anderson GP: Avidin: a natural bridge for quantum dot-antibody conjugates. J Am Chem Soc 2002, 124(22):6378–6382. 10.1021/ja0125570
Jaiswal JK, Mattoussi H, Mauro JM, Simon SM: Use of quantum dots for live cell imaging. Nat Meth 2004, 1(1):6. 10.1038/nmeth1004-6
Jaiswal JK, Goldman ER, Mattoussi H, Simon SM: Long-term multiple color imaging of live cells using quantum dot bioconjugates. Nat Biotech 2003, 21(1):47–51. 10.1038/nbt767
Hasegawa U, Nomura SM, Kaul SC: Nanogel-quantum dot hybrid nanoparticles for live cell imaging. Biochem Biophys Res Commun 2005, 331(4):917–921. 10.1016/j.bbrc.2005.03.228
Leevy WM, Lambert TN, Johnson JR, Morris J, Smith BD: Quantum dot probes for bacteria distinguish Escherichia coli mutants and permit in vivo imaging. Chem Commun 2008, 20: 2331–2333.
Tallury P, Malhotra A, Byrne LM, Santra S: Nanobioimaging and sensing of infectious diseases. Adv Drug Deliv Rev 2010, 62(4–5):424–437.
Su X-L, Li Y: Quantum dot biolabeling coupled with immunomagnetic separation for detection of Escherichia coli O157:H7. Anal Chem 2004, 76(16):4806–4810. 10.1021/ac049442+
Yang L, Li Y: Simultaneous detection of Escherichia coli O157[ratio]H7 and Salmonella Typhimurium using quantum dots as fluorescence labels. Analyst 2006, 131(3):394–401. 10.1039/b510888h
Chang Y-P, Pinaud F, Antelman J, Weiss S: Tracking bio-molecules in live cells using quantum dots. J Biophotonics 2008, 1(4):287–298. 10.1002/jbio.200810029
Cherry RJ: Keeping track of cell surface receptor. Trends Cell Biol 1992, 2(8):242–244. 10.1016/0962-8924(92)90312-B
Saxton MJ, Jacobson K: Single-particle tracking: applications to membrane dynamics. Annu Rev Biophys Biomol Struct 1997, 26: 373–399. 10.1146/annurev.biophys.26.1.373
Howarth M: Monovalent, reduced-size quantum dots for imaging receptors on living cells. Nat Methods 2008, 5(5):397–399. 10.1038/nmeth.1206
Chen H: Altered membrane dynamics of quantum dot-conjugated integrins during osteogenic differentiation of human bone marrow derived progenitor cells. Biophys J 2007, 92(4):1399–1408. 10.1529/biophysj.106.094896
Haggie PM: Tracking of quantum dot-labeled CFTR shows near immobilization by C-terminal PDZ interactions. Mol Biol Cell 2006, 17(12):4937–4945. 10.1091/mbc.E06-08-0670
Crane JM, Verkman AS: Long-range nonanomalous diffusion of quantum dot-labeled aquaporin-1 water channels in the cell plasma membrane. Biophys J 2008, 94(2):702–713. 10.1529/biophysj.107.115121
Bouzigues C: Asymmetric redistribution of GABA receptors during GABA gradient sensing by nerve growth cones analyzed by single quantum dot imaging. Proc Natl Acad Sci 2007, 104(27):11251–11256. 10.1073/pnas.0702536104
Dahan M: Diffusion dynamics of glycine receptors revealed by single-quantum dot tracking. Science 2003, 302(5644):442–445. 10.1126/science.1088525
Roullier V: High-affinity labeling and tracking of individual histidine-tagged proteins in live cells using Ni2+ tris-nitrilotriacetic acid quantum dot conjugates. Nano Lett 2009, 9(3):1228–1234. 10.1021/nl9001298
Lidke DS: Quantum dot ligands provide new insights into erbB/HER receptor-mediated signal transduction. Nat Biotechnol 2004, 22(2):198–203. 10.1038/nbt929
Watanabe TM, Higuchi H: Stepwise movements in vesicle transport of HER2 by motor proteins in living cells. Biophys J 2007, 92(11):4109–4120. 10.1529/biophysj.106.094649
Groc L: Differential activity-dependent regulation of the lateral mobilities of AMPA and NMDA receptors. Nat Neurosci 2004, 7(7):695–696. 10.1038/nn1270
Choquet D, Triller A: The role of receptor diffusion in the organization of the postsynaptic membrane. Nat Rev Neurosci 2003, 4(4):251–265.
Xiao Y, Barker PE: Semiconductor nanocrystal probes for human metaphase chromosomes. Nucleic Acids Res 2004, 32(3):e28-e28. 10.1093/nar/gnh024
Pierobon P: Velocity, processivity, and individual steps of single myosin V molecules in live cells. Biophys J 2009, 96(10):4268–4275. 10.1016/j.bpj.2009.02.045
Nelson SR: Random walk of processive, quantum dot-labeled myosin Va molecules within the actin cortex of COS-7 cells. Biophys J 2009, 97(2):509–518. 10.1016/j.bpj.2009.04.052
Yoo J: Intracellular imaging of targeted proteins labeled with quantum dots. Exp Cell Res 2008, 314(19):3563–3569. 10.1016/j.yexcr.2008.09.014
Courty S: Tracking individual kinesin motors in living cells using single quantum-dot imaging. Nano Lett 2006, 6(7):1491–1495. 10.1021/nl060921t
Ishido M, Kasuga N: In situ real-time imaging of the satellite cells in rat intact and injured soleus muscles using quantum dots. Histochem Cell Biol 2011, 135(1):21–26. 10.1007/s00418-010-0767-x
Stylianou P, Skourides PA: Imaging morphogenesis, in Xenopus with quantum dot nanocrystals. Mech Dev 2009, 126(10):828–841. 10.1016/j.mod.2009.07.008
Navarro DA, Bisson MA, Aga DS: Investigating uptake of water-dispersible CdSe/ZnS quantum dot nanoparticles by Arabidopsis thaliana plants. J Hazard Mater 2012, 211–212: 427–435.
Gautrot JE, Zhu XX: Macrocyclic bile acids: from molecular recognition to degradable biomaterial building blocks. J Mater Chem 2009, 19(32):5705–5716. 10.1039/b821340b
Lim IIS: Gold and magnetic oxide/gold core/shell nanoparticles as bio-functional nanoprobes. Nanotechnology 2008, 19(30):305102. 10.1088/0957-4484/19/30/305102
Duan H, Nie S: Cell-penetrating quantum dots based on multivalent and endosome-disrupting surface coatings. J Am Chem Soc 2007, 129(11):3333–3338. 10.1021/ja068158s
Smith AM: Bioconjugated quantum dots for in vivo molecular and cellular imaging. Adv Drug Deliv Rev 2008, 60(11):1226–1240. 10.1016/j.addr.2008.03.015
Wang L: Core@shell nanomaterials: gold-coated magnetic oxide nanoparticles. J Mater Chem 2008, 18(23):2629–2635. 10.1039/b719096d
Park K: New generation of multifunctional nanoparticles for cancer imaging and therapy. Adv Funct Mater 2009, 19(10):1553–1566. 10.1002/adfm.200801655
Wu W: In-situ immobilization of quantum dots in polysaccharide-based nanogels for integration of optical pH-sensing, tumor cell imaging, and drug delivery. Biomaterials 2010, 31(11):3023–3031. 10.1016/j.biomaterials.2010.01.011
Lee KH: Quantitative molecular profiling of biomarkers for pancreatic cancer with functionalized quantum dots. Nanomedicine in press in press
Gokarna A: Quantum dot-based protein micro- and nanoarrays for detection of prostate cancer biomarkers. Proteomics 2008, 8(9):1809–1818. 10.1002/pmic.200701072
Liu J: Multiplexed detection and characterization of rare tumor cells in Hodgkin's lymphoma with multicolor quantum dots. Anal Chem 2010, 82(14):6237–6243. 10.1021/ac101065b
Ray S: Emerging nanoproteomics approaches for disease biomarker detection: A current perspective. J Proteomics 2011, 74(12):2660–2681. 10.1016/j.jprot.2011.04.027
Yu Y: Hydrothermal synthesis of GSH–TGA co-capped CdTe quantum dots and their application in labeling colorectal cancer cells. Colloids Surf B Biointerfaces 2012, 95: 247–253.
American Cancer Society: Pancreatic cancer. http://www.cancer.org/cancer/pancreaticcancer/detailedguide/pancreatic-cancer-key-statistics http://www.cancer.org/cancer/pancreaticcancer/detailedguide/pancreatic-cancer-key-statistics
Resch-Genger U: Quantum dots versus organic dyes as fluorescent labels. Nat Meth 2008, 5(9):763–775. 10.1038/nmeth.1248
Tripp RA: Bioconjugated nanoparticle detection of respiratory syncytial virus infection. Int J Nanomedicine 2007, 2(1):117–124. 10.2147/nano.2007.2.1.117
Agrawal A: Counting single native biomolecules and intact viruses with color-coded nanoparticles. Anal Chem 2006, 78(4):1061–1070. 10.1021/ac051801t
Bentzen EL: Progression of respiratory syncytial virus infection monitored by fluorescent quantum dot probes. Nano Lett 2005, 5(4):591–595. 10.1021/nl048073u
Dwarakanath S: Quantum dot-antibody and aptamer conjugates shift fluorescence upon binding bacteria. Biochem Biophys Res Commun 2004, 325(3):739–743. 10.1016/j.bbrc.2004.10.099
Goldman ER: Multiplexed toxin analysis using four colors of quantum dot fluororeagents. Anal Chem 2003, 76(3):684–688.
Zhao Y: Simultaneous detection of multifood-borne pathogenic bacteria based on functionalized quantum dots coupled with immunomagnetic separation in food samples. J Agric Food Chem 2008, 57(2):517–524.
Hahn MA, Tabb JS, Krauss TD: Detection of single bacterial pathogens with semiconductor quantum dots. Anal Chem 2005, 77(15):4861–4869. 10.1021/ac050641i
Mukhopadhyay B: Bacterial detection using carbohydrate-functionalised CdS quantum dots: a model study exploiting E. coli recognition of mannosides. Tetrahedron Lett 2009, 50(8):886–889. 10.1016/j.tetlet.2008.12.029
Edgar R: High-sensitivity bacterial detection using biotin-tagged phage and quantum-dot nanocomplexes. Proc Natl Acad Sci U S A 2006, 103(13):4841–4845. 10.1073/pnas.0601211103
Zhu L, Ang S, Liu W-T: Quantum dots as a novel immunofluorescent detection system for Cryptosporidium parvum and Giardia lamblia. Appl Environ Microbiol 2004, 70(1):597–598. 10.1128/AEM.70.1.597-598.2004
Klostranec JM, Xiang Q, Farcas GA: Convergence of quantum dot barcodes with microfluidics and signal processing for multiplexed high-throughput infectious disease diagnostics. Nano Lett 2007, 7(9):2812–2818. 10.1021/nl071415m
Gazouli M, Liandris E, Andreadou M, Sechi LA, Masala S, Paccagnini D, Ikonomopoulos J: Specific detection of unamplified mycobacterial DNA by use of fluorescent semiconductor quantum dots and magnetic beads. J Clin Microbiol 2010, 48(8):2830–2835. 10.1128/JCM.00185-10
Griffith DE: An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007, 175(4):367–416. 10.1164/rccm.200604-571ST
Veigas B, Doria G, Baptista PV: Nanodiagnostics for tuberculosis. In Understanding Tuberculosis - Global Experiences and Innovative Approaches to the Diagnosis. Edited by: Cardona PJ. Rijeka: InTech; 2012:20.
GhoshMitra S: Role of engineered nanocarriers for axon regeneration and guidance: current status and future trends. Adv Drug Deliv Rev 2012, 64(1):110–125. 10.1016/j.addr.2011.12.013
Langlois JA, Rurland-Brown W, Thomas KE: Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths. Atlanta, GA: Dept. of Health and Human Services (US), Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2004.
National Spinal Cord Injury Statistical Center: Facts and figures at a glance. http://www.nscisc.uab.edu/public_content/pdf/Facts%202011%20Feb%20Final.pdf 2011 http://www.nscisc.uab.edu/public_content/pdf/Facts%202011%20Feb%20Final.pdf 2011
Pathak S: Quantum dot applications to neuroscience: new tools for probing neurons and glia. J Neurosci 2006, 26(7):1893–1895. 10.1523/JNEUROSCI.3847-05.2006
Alivisatos P: The use of nanocrystals in biological detection. Nat Biotech 2004, 22(1):47–52. 10.1038/nbt927
Smith A: Engineering luminescent quantum dots for in vivo molecular and cellular imaging. Ann Biomed Eng 2006, 34(1):3–14. 10.1007/s10439-005-9000-9
Gao X: In vivo cancer targeting and imaging with semiconductor quantum dots. Nat Biotech 2004, 22(8):969–976. 10.1038/nbt994
Vu TQ: Peptide-conjugated quantum dots activate neuronal receptors and initiate downstream signaling of neurite growth. Nano Lett 2005, 5(4):603–607. 10.1021/nl047977c
Sundara Rajan S, Vu TQ: Quantum dots monitor TrkA receptor dynamics in the interior of neural PC12 cells. Nano Lett 2006, 6(9):2049–2059. 10.1021/nl0612650
Howarth M: Targeting quantum dots to surface proteins in living cells with biotin ligase. Proc Natl Acad Sci USA 2005, 102(21):7583–7588. 10.1073/pnas.0503125102
Prasad B: Long-term exposure of CdTe quantum dots on PC12 cellular activity and the determination of optimum non-toxic concentrations for biological use. J Nanobiotechnology 2010, 8(1):7. 10.1186/1477-3155-8-7
WHO: World Malaria Report 2010. Geneva: WHO; 2010.
Ku MJ: Quantum dots: a new tool for anti-malarial drug assays. Malar J 2011, 10: 118. 10.1186/1475-2875-10-118
Tokumasu F: Band 3 modifications in Plasmodium falciparum-infected AA and CC erythrocytes assayed by autocorrelation analysis using quantum dots. J Cell Sci 2005, 118(Pt 5):1091–1098.
Wu D, Chen Z: ZnS quantum dots as pH probes for study of enzyme reaction kinetics. Enzyme Microb Technol 2012, 51(1):47–52. 10.1016/j.enzmictec.2012.03.012
Jin WJ: Photoactivated luminescent CdSe quantum dots as sensitive cyanide probes in aqueous solutions. Chem Commun (Camb) 2005. 10.1039/B414858D
Xie H-Y: Luminescent CdSe-ZnS quantum dots as selective Cu2+ probe. Spectrochim Acta A Mol Biomol Spectrosc 2004, 60(11):2527–2530. 10.1016/j.saa.2003.12.039
Tomasulo M, Yildiz I, Raymo FM: pH-sensitive quantum dots. J Phys Chem B 2006, 110(9):3853–3855. 10.1021/jp060185h
Sun YH: Photostability and pH sensitivity of CdSe/ZnSe/ZnS quantum dots in living cells. Nanotechnology 2006, 17(17):4469. 10.1088/0957-4484/17/17/031
Wang Y-Q: Cadmium telluride quantum dots as pH-sensitive probes for tiopronin determination. Anal Chim Acta 2008, 610(1):50–56. 10.1016/j.aca.2008.01.015
Liu Y-S: pH-Sensitive photoluminescence of CdSe/ZnSe/ZnS quantum dots in human ovarian cancer cells. J Phys Chem C 2007, 111(7):2872–2878. 10.1021/jp0654718
Yun Z: Using cadmium telluride quantum dots as a proton flux sensor and applying to detect H9 avian influenza virus. Anal Biochem 2007, 364(2):122–127. 10.1016/j.ab.2007.02.031
Deng Z: Green and orange CdTe quantum dots as effective ph-sensitive fluorescent probes for dual simultaneous and independent detection of viruses. J Phys Chem B 2007, 111(41):12024–12031. 10.1021/jp074609z
Gao M, Kirstein S: Mo1hwald H: Strongly photoluminescent CdTe nanocrystals by proper surface modification. J Phys Chem B 1998, 102(43):8360–8363. 10.1021/jp9823603
Lueking A: Protein microarrays for gene expression and antibody screening. Anal Biochem 1999, 270(1):103–111. 10.1006/abio.1999.4063
MacBeath G, Schreiber SL: Printing proteins as microarrays for high-throughput function determination. Science 2000, 289(5485):1760–1763.
Hergenrother PJ, Depew KM, Schreiber SL: Small-molecule microarrays: covalent attachment and screening of alcohol-containing small molecules on glass slides. J Am Chem Soc 2000, 122(32):7849–7850. 10.1021/ja0014032
Nelson RW, Nedelkov D, Tubbs KA: Biosensor chip mass spectrometry: a chip-based proteomics approach. Electrophoresis 2000, 21(6):1155–1163. 10.1002/(SICI)1522-2683(20000401)21:6<1155::AID-ELPS1155>3.0.CO;2-X
Schweitzer B, Kingsmore SF: Measuring proteins on microarrays. Curr Opin Biotechnol 2002, 13(1):14–19. 10.1016/S0958-1669(02)00278-1
Sun B: Microminiaturized immunoassays using quantum dots as fluorescent label by laser confocal scanning fluorescence detection. J Immunol Methods 2001, 249(1–2):85–89.
Shingyoji M: Quantum dots-based reverse phase protein microarray. Talanta 2005, 67(3):472–478. 10.1016/j.talanta.2005.06.064
Kerman K: Quantum dot-based immunosensor for the detection of prostate-specific antigen using fluorescence microscopy. Talanta 2007, 71(4):1494–1499. 10.1016/j.talanta.2006.07.027
Geho D: Pegylated, steptavidin-conjugated quantum dots are effective detection elements for reverse-phase protein microarrays. Bioconjug Chem 2005, 16(3):559–566. 10.1021/bc0497113
Zajac A: Protein microarrays and quantum dot probes for early cancer detection. Colloids Surf B Biointerfaces 2007, 58(2):309–314. 10.1016/j.colsurfb.2007.02.019
Stephens DJ, Allan VJ: Light microscopy techniques for live cell imaging. Science 2003, 300(5616):82–86. 10.1126/science.1082160
Weijer CJ: Visualizing signals moving in cells. Science 2003, 300(5616):96–100. 10.1126/science.1082830
Liu BR: Intracellular delivery of quantum dots mediated by a histidine- and arginine-rich HR9 cell-penetrating peptide through the direct membrane translocation mechanism. Biomaterials 2011, 32(13):3520–3537. 10.1016/j.biomaterials.2011.01.041
Zhou M, Ghosh I: Quantum dots and peptides: a bright future together. Pept Sci 2007, 88(3):325–339. 10.1002/bip.20655
Dixit SK: Quantum dot encapsulation in viral capsids. Nano Lett 2006, 6(9):1993–1999. 10.1021/nl061165u
Jia N: Intracellular delivery of quantum dots tagged antisense oligodeoxynucleotides by functionalized multiwalled carbon nanotubes. Nano Lett 2007, 7(10):2976–2980. 10.1021/nl071114c
Chen B: Transmembrane delivery of the cell-penetrating peptide conjugated semiconductor quantum dots. Langmuir 2008, 24(20):11866–11871. 10.1021/la802048s
Xue F: Enhancement of intracellular delivery of cdte quantum dots (qds) to living cells by tat conjugation. J Fluoresc 2007, 17(2):149–154. 10.1007/s10895-006-0152-2
Delehanty JB: Self-assembled quantum dot−peptide bioconjugates for selective intracellular delivery. Bioconjug Chem 2006, 17(4):920–927. 10.1021/bc060044i
Ruan G: Imaging and tracking of Tat peptide-conjugated quantum dots in living cells: new insights into nanoparticle uptake, intracellular transport, and vesicle shedding. J Am Chem Soc 2007, 129(47):14759–14766. 10.1021/ja074936k
Wei Y: Surface coating directed cellular delivery of TAT-functionalized quantum dots. Bioconjug Chem 2009, 20(9):1752–1758. 10.1021/bc8003777
Lagerholm BC: Multicolor coding of cells with cationic peptide coated quantum dots. Nano Lett 2004, 4(10):2019–2022. 10.1021/nl049295v
Bagalkot V: Quantum dot−aptamer conjugates for synchronous cancer imaging, therapy, and sensing of drug delivery based on bi-fluorescence resonance energy transfer. Nano Lett 2007, 7(10):3065–3070. 10.1021/nl071546n
Bakalova R: Multimodal silica-shelled quantum dots: direct intracellular delivery, photosensitization, toxic, and microcirculation effects. Bioconjug Chem 2008, 19(6):1135–1142. 10.1021/bc700431c
Yum K: Mechanochemical delivery and dynamic tracking of fluorescent quantum dots in the cytoplasm and nucleus of living cells. Nano Lett 2009, 9(5):2193–2198. 10.1021/nl901047u
Yuan Q, Hein S, Misra RDK: New generation of chitosan-encapsulated ZnO quantum dots loaded with drug: synthesis, characterization and in vitro drug delivery response. Acta Biomater 2010, 6(7):2732–2739. 10.1016/j.actbio.2010.01.025
Zhang P, Liu W: ZnO QD@PMAA-co-PDMAEMA nonviral vector for plasmid DNA delivery and bioimaging. Biomaterials 2010, 31(11):3087–3094. 10.1016/j.biomaterials.2010.01.007
Jablonski AE, Humphries WH, Payne CK: Pyrenebutyrate-mediated delivery of quantum dots across the plasma membrane of living cells. J Phys Chem B 2008, 113(2):405–408.
Qi L, Gao X: Quantum dot−amphipol nanocomplex for intracellular delivery and real-time imaging of siRNA. ACS Nano 2008, 2(7):1403–1410. 10.1021/nn800280r
Yan M: An in vitro study of vascular endothelial toxicity of CdTe quantum dots. Toxicology 2011, 282(3):94–103. 10.1016/j.tox.2011.01.015
Chen N: The cytotoxicity of cadmium-based quantum dots. Biomaterials 2012, 33(5):1238–1244. 10.1016/j.biomaterials.2011.10.070
Akbarzadeh A, Asgari D, Zarghami N, Mohammad R, Davaran S: Preparation and in vitro evaluation of doxorubicin-loaded Fe3O4magnetic nanoparticles modified with biocompatible co-polymers. Int J Nanomedicine 2012, 7: 511–526.
Akbarzadeh A, Zarghami N, Mikaeili H, Asgari D, Goganian AM, Khiabani HK, Samiei M, Davaran S: Synthesis, characterization, and in vitro evaluation of novel polymer-coated magnetic nanoparticles for controlled delivery of doxorubicin. Nanotechnol Sci Appl 2012, 5: 13–25.
Akbarzadeh A, Samiei M, Davaran S: Magnetic nanoparticles: preparation, physical properties, and applications in biomedicine. Nanoscale Res Lett 2012, 7: 144. 10.1186/1556-276X-7-144
Wang ZM, Kunets VP, Xie YZ, Schmidbauer M, Dorogan VG, Mazur YI, Salamo GJ: Multilayer self-organization of InGaAs quantum wires on GaAs surfaces. Phys. Lett. A 2010, 375: 170–173. 10.1016/j.physleta.2010.10.051
Passmore BS, Wu J, Manasreh MO, Kunets VP, Lytvyn PM, Salamo GJ: Room temperature near-infrared photoresponse based on interband transition in In 0.35 Ga 0.35 As multiple quantum dot photodetector. IEE Electron Device Letters 2008, 29: 224–227.
Hardman R: A toxicologic review of quantum dots: toxicity depends on physicochemical and environmental factors. Environ Health Perspect 2006, 114(2):165–172. 10.1289/ehp.8284
Acknowledgments
The authors are grateful to the financial support from the Department of Medical Nanotechnology, Faculty of Advanced Medical Science, Iran, National Science Foundation Tabriz, Iran, and the Drug Applied Research Center Tabriz University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
SD conceived of the study and participated in its design and coordination. AA participated in the sequence alignment and drafted the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Valizadeh, A., Mikaeili, H., Samiei, M. et al. Quantum dots: synthesis, bioapplications, and toxicity. Nanoscale Res Lett 7, 480 (2012). https://doi.org/10.1186/1556-276X-7-480
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1556-276X-7-480