Abstract
Electrochromic switching devices have elicited considerable attention because these thin films are among the most promising materials for energy-saving applications. The vanadium oxide system is simple and inexpensive because only a single-layer film of this material is sufficient for coloration. Vanadium dioxide thin films are fabricated by electrochemical deposition and cyclic voltammetry. Chronoamperometric analyses have indicated that the thin V2O5 film demonstrates faster intercalation and deintercalation of lithium ions than those of the thick V2O5 film, benefiting the coloration rate. Despite substantial research on the synthesis of vanadium oxides, the monitoring of electronic and atomic structures during growth and coloration of such material has not been thoroughly examined. In the present study, in situ X-ray absorption spectroscopy (XAS) is employed to determine the electronic and atomic structures of V2O5 thin films during electrochemical growth and then electrochromic coloration. In situ XAS results demonstrate the growth mechanism of the electrodeposited V2O5 thin film and suggest that its electrochromic performance strongly depends on the local atomic structure. This study improves our understanding of the electronic and atomic properties of the vanadium oxide system grown by electrochemical deposition and enhances the design of electrochromic materials for potential energy-saving applications.
Similar content being viewed by others
Background
The recent years have witnessed growing environmental concerns and increasing energy demands [1]. In the USA, up to 40 % energy is used for primary energy consumption of buildings, which contribute over 30 % CO2 emissions [2]. Thus, the effective use of energy has increasingly become an important issue. Smart windows can change optical properties by using an applied electric field or current, thereby avoiding excessive solar heating while taking advantage of heating mechanisms when necessary. Vanadium oxide systems comprise many oxide phases, including VO, V2O3, VO2, V6O13, V3O7, and V2O5. In particular, vanadium pentoxide is the most stable oxide in such systems. V2O5 exhibits an energy gap of approximately 2.2 eV and undergoes semiconductor–metal transition at around 250 °C. V2O5 materials demonstrate a color change when lithium ions are injected into or extracted from the layer spaces. Hence, V2O5 materials provide significant potential for applications in energy-saving devices, such as catalysts, sensors, electronic materials, and battery electrodes [3–5]. V2O5 films have been fabricated by various methods, such as reactive DC magnetron sputtering [6], vacuum-evaporated deposition [7], chemical vapor deposition [8], sol–gel method [9], spray pyrolysis technique [10], and electrodeposition [11]. Electrochemical method is greatly advantageous over other methods in terms of economics and flexibility; typically, this method can also be used to fabricate mesostructured thin films [12]. The high surface area of the mesoporous structure of V2O5 thin films provides porous channels, which facilitate fast ion diffusion and effective strain relaxation upon cycling of Li ion intercalation and deintercalation. Numerous studies on the application of V2O5 in energy-saving (smart windows [13]) or energy storage devices (batteries/supercapacitors [14]) have reported various synthesis methods [15], electrochemical properties, and photocatalytic performances [16]. Several studies have also demonstrated the importance of electronic structure characterization [17–19]. Eyert et al. [17] indicated that octahedral distortions enhance the optical band gap. Willinger et al. [18] compared the basic structural VO5 units in α-V2O5 and γ-V2O5 with regard to the differences in their geometric and electronic structures. The relation between geometric and electronic structures is critical because the basic structural unit is also common to industrial vanadium phosphorus oxide (VPO) catalysts [18]. However, the detail determination of the local atomic/electronic structure during growth of such electrochemically deposited film and during electrochromic coloration has not been thoroughly examined because of the lack of proper characterization tools. In the current study, an in situ electrochemical liquid cell was built, and in situ synchrotron hard X-ray absorption spectroscopy (XAS) was performed to monitor the electronic and atomic structures during the electrochemical deposition of V2O5 nano thin films. Changes in atomic/electronic structure upon coloration were determined. This approach is critical in determining the electrochemical growth and coloration mechanism, providing a great opportunity to further understand and improve the electrochemical properties of electrochromic materials.
Methods
Characterization and Measurement of Electrochemical Properties
Scanning electron microscopy (SEM, model PHILIPS S-4300) was used to examine the surface morphology. Electrodeposition was performed on a potentiostat/galvanostat (Princeton Applied Research, Versa STAT 4). A conventional three-electrode cell was utilized with an ITO substrate. V2O5 thin films were electrodeposited to a 1:1 mixture of deionized water and ethanol, which contained 1 M VOSO4·xH2O. Electrodepositions were performed at −0.7 V against the reference electrode for 20, 40, and 60 s. The lithium-ion intercalation/deintercalation properties of the V2O5 material were investigated in 1 M LiClO4 solution containing propylene carbonate. Cyclic voltammetry was performed between −1 and +1.25 V (versus Pt) at a scanning rate of 0.025 V s−1 using a potentiostat/galvanostat (Princeton Applied Research, Versa STAT 4). Chronoamperometric measurements were obtained at a constant voltage of +0.5 V, and the current change was monitored for 45 s at room temperature.
Measurement of Electronic Structure
V K-edge XAS spectra were collected at the hard X-ray beamline BL17C of the NSRRC. Electrodeposition of the V2O5 electrodes was performed potentiostatically at 0.7 V at room temperature using Au-coated Si3N4 as the substrate in the in situ reaction cell. Pt electrodes were used as the counter and reference electrodes. Figure 1 presents a schematic of the substrate-film-sample holder assembly in contact with the VOSO4·xH2O and LiClO4 electrolyte. The total fluorescence yield signal was collected with this sample holder. The samples were deposited onto a Si3N4 membrane window and used as working electrodes in a three-electrode setup. Prior to the deposition of the V2O5 films, 10-nm Au layers were evaporated on the Si3N4 window to provide an adhesive metallic current collector between V2O5 and Si3N4. The Si3N4 membrane window is transparent for X-rays; the membrane is attached to a PVC supporting frame by an Araldite® adhesive that creates a tight seal.
Results and Discussion
Figure 2 shows SEM which examines the surface morphology of the electrochemical growth of the V2O5 thin films. The V2O5 thin films that were deposited for 20 s were relatively smooth and adhered very well onto the substrate, as shown in Fig. 2a. As the deposition time increased to 40 and 60 s, the morphology of the V2O5 thin films significantly changed (Fig. 2b, c). The morphology of the V2O5 thin films changed from smooth to typical sea-island morphology. The film thickness of the electrochemical growth of the V2O5 thin films was measured using the cross-sectional SEM images (inset of Fig. 2a–c), with thicknesses of 321, 621, and 1047 nm for the deposition times of 20, 40, and 60 s, respectively.
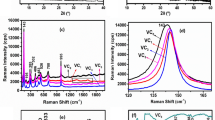
In situ K-edge XAS was employed to investigate the electrochemical growth of the V2O5 thin films in both local electronic and atomic structures as a function of time. Figure 3 shows a comparison of the normalized V K-edge spectra for the V2O5 films deposited at different times (20, 40, and 60 s). Numerous reports dealing with XAS spectra of V2O5 have already been published. The first feature shown in the spectra is the pre-edge peak (a). The intensity and energy position of the pre-edge may be used qualitatively to derive structural and chemical information. Pre-edge absorption is associated with dipole forbidden s → d transitions, which become allowed in the vanadium 3D states mixed with oxygen p states arising from the noncentrosymmetric environment of the slightly distorted octahedral [20]. Hence, the pre-edge peak intensity is very sensitive to alterations in the local geometrical symmetry [21]. Features b and c originate from the 1s core-electron excitation to the 4p orbital. The above features are highly sensitive to the effective valence state of the vanadium and the chemical environment surrounding the vanadium site [22, 23]. The V2O5 thin films deposited for 20 s are most likely in the form of V5+ with octahedral symmetry [19, 23]. Examination of the spectra with deposition time reveals that the edge energy shifted to increased values (inset of Fig. 3a), suggesting that vanadium is most likely in the form of V5+ with pyramid symmetry in the V2O5 electrode deposited for 40 and 60 s [19, 24]. In addition to the shift in edge energy, the intensity of the pre-edge peak also increases, suggesting an increase in the distortion of the VO5 square pyramid with increasing deposition time [25].
The electrochemical behavior of the V2O5 material was investigated using the three-electrode system, in which Pt foils were used as the counter and reference electrodes. Cyclic voltammetry and chronoamperometry were adopted to investigate the Li insertion/extraction behavior in 1 M LiClO4 propylene carbonate solution with a scan rate of 0.25 V s−1. Figure 4a shows typical cyclic voltammograms of V2O5 electrodes with different deposition times in the potential range of −1 to 1.25 V. The electrochemical Li+ insertion process occurring at V2O5 electrodes can be expressed by V2O5 + x Li+ + xe− ↔ LixV2O5 [14, 26], which is accompanied by color changes. These processes lead to film coloration [yellow → green → blue (inset of Fig. 4c)]. The V2O5 electrode shows two sets of broad, symmetric, and well-separated redox peaks, indicating sluggish lithium ion insertion/deinsertion kinetics. Moreover, compared with the V2O5 electrode at 40 and 60 s deposition times, all the peaks from the V2O5 electrode at 20 s deposition time shifted to decreased potentials (Fig. 4a). The average voltage difference between the cathodic and the corresponding anodic double peaks of the redox reactions (Fig. 4b) decreases from 350 mV at 60 s deposition time to 175 mV at 20 s deposition time, indicating easier intercalation and deintercalation of lithium ions. To compare the coloration efficiencies directly, the V2O5 electrodes at different deposition times were first biased at −0.5 V versus a Pt reference electrode for 45 s to facilitate lithium intercalation. The polarity was then switched immediately to +0.5 V to initiate lithium deintercalation, recording the change in current (Fig. 4b). Compared with the V2O5 electrode deposited for 20 s, the V2O5 electrode deposited for 40 and 60 s showed a very slow current decay, indicating that the charge characteristics, such as deposition times, are much slower in cation deintercalation. In the case of the V2O5 electrode deposited for 20 s, the initial increase in current was much higher than that of the V2O5 electrodes deposited for 40 and 60 s. This finding implies that the V2O5 electrode deposited for 20 s indicates faster intercalation and deintercalation of lithium ions. The higher kinetics of lithium insertion/extraction in the V2O5 electrode deposited for 20 s depends not only on the film thickness of the electrode but also on the electronic and atomic structures, as revealed in greater detail by the in situ K-edge XAS discussed later.
In situ XAS at V K-edge was performed during the coloration process upon the electrochemical reaction to gain insights into the effects of delithiation/lithiation on the oxidation states of vanadium, as well as the local atomic structure of the V2O5 thin films. Although the thinner film (deposited for 20 s) exhibits faster coloration rate than a thicker film (deposited for 60 s), the thicker film is more stable than the thinner one. Thus, the XAS spectrum of a V2O5 film deposited for 60 s is shown in Fig. 5. The insets compare the pre-peak intensities during delithiation (left panel) and lithiation (right panel) processes. Notably, two major changes under bias potential from 0 to −0.6 V caused by Li-ion intercalation in the spectral features, which were observed in the pre-edge region, were indicated as follows: a shift of pre-edge peak position to the lower energy and a decrease in pre-edge peaks. The shift to low energy was attributed to the decreased oxidation state of metal ions because of Li-ion intercalation, which can be conceptually caused by the reduced effective nuclear charge of the metal ions with decreased oxidation state. The pre-edge peak intensity clearly decreased for the bias potentials from 0 to −0.6 V, suggesting that the local structure around the V atom became more symmetrical with Li-ion intercalation. However, extracting lithium under a bias potential from −0.6 to +0.6 V changes the color of the film from deep blue to green to yellow. At −0.3, 0, and 0.6 V, the pre-edge peak of the V K-edge shifts to high energy when the film was oxidized, and partial V4+ ions change into V5+. The remaining V4+ ions reduced to V5+ at 0.6 V. The positive shift in the V K-edge indicates that the average oxidation state of V increased because of an increase in the attractive potential of the nucleus. Additionally, the increase in the pre-edge peak area corresponds to the gradually decreasing lithium content, revealing that structural symmetry was modified from Oh (V4+) to a mixture of Py (V5+). Without the in situ electrochemical cell, the above atomic/electronic structure information cannot be obtained. Overall, these results emphasize the importance of in situ X-ray spectroscopic characterization on the atomic/electronic structures of energy materials in their working condition.
Conclusions
Electrochromic switching devices have been widely investigated because such switching can control the throughput of visible light and solar radiation into buildings by applying electrical voltage, thereby imparting energy efficiency. Electrochemical in situ XAS studies indicated that the electrodeposition of V2O5 under constant 0.7 V conditions produced V2O5 thin films with different local electronic and atomic structures along with variation of film thickness. Given the increasing film thickness, the local geometrical symmetry of V2O5 thin films varies from V5+ with octahedral symmetry to V5+ with pyramid symmetry. In situ XAS was performed in the present study to monitor the effects of delithiation/lithiation on the vanadium oxidation states and the local atomic structures of the V2O5 thin films. Color switching upon the intercalation of hydrogen is caused by the valence change of the cations. Such effect is accompanied by structural rearrangement. This in situ XAS electrochemical cell setup allows real-time monitoring of the element-specific electronic structural changes in a system at all stages of electrochemical reaction. It is anticipated that using this technique, the growth parameters of V2O5 thin films can be fine-tuned, achieving optimization.
References
Luo J, Im JH, Mayer MT, Schreier M, Nazeeruddin MK, Park NG et al. (2014) Water photolysis at 12.3 % efficiency via perovskite photovoltaics and Earth-abundant catalysts. Science 345:1593
Yang L, Yan H, Lam Joseph C (2014) Thermal comfort and building energy consumption implications–a review. Appl Energy 115:164
Fujita Y, Miyazaki K, Tatsuyama C (1985) On the electrochromism of evaporated V2O5 films. Jpn J Appl Phys 24:1082
Wang Z, Chen J, Hu X (2000) Electrochromic properties of aqueous sol-gel derived vanadium oxide films with different thickness. Thin Solid Films 375:238
Karimov KS, Saleem M, Mahroof-Tahir M, Akram R, Saeed Chanee MT, Niaz AK (2014) Resistive humidity sensor based on vanadium complex films. J Semicond 9:094001
Baddour-Hadjean R, Pereira-Ramos JP, Navone C, Smirnov M (2008) Raman microspectrometry study of electrochemical lithium intercalation into sputtered crystalline V2O5 thin films. Chem Mater 20:1916
Rajendra Kumar RT, Karunagaran B, Venkatachalam S, Mangalaraj D, Narayandass SK, Kesavamoorthy R (2003) Influence of deposition temperature on the growth of vacuum evaporated V2O5 thin films. Mater Lett 57:3820
Davide B, Lidia A, Federico C, Vito Di N, Andrea G, Gian Andrea R et al (2000) Highly oriented V2O5 nanocrystalline thin films by plasma-enhanced chemical vapor deposition. Chem Mater 12:98
Patrissi Charles J, Martin Charles R (1999) Sol-gel-based template synthesis and li-insertion rate performance of nanostructured vanadium pentoxide. J Electrochem Soc 146:3176
Mousavi M, Kompany A, Shahtahmasebi N, Bagheri-Mohagheghi MM (2013) The effect of solution concentration on the physical and electrochemical properties of vanadium oxide films deposited by spray pyrolysis. J Semicond 10:103001
Arunabha G, Eun Ju R, Meihua J, Hae-Kyung J, Tae Hyung K, Chandan B et al. (2011) High pseudocapacitance from ultrathin V2O5 films electrodeposited on self‐standing carbon‐nanofiber paper. Adv Funct Mater 21:2541
Liu P, Lee S-H, Tracy CE, Yan Y, Turner JA (2002) Preparation and lithium insertion properties of mesoporous vanadium oxide. Adv Mater 14:27
Takahashi K, Wang Y, Cao G (2005) Growth and electrochromic properties of single-crystal V2O5 nanorod arrays. Appl Phys Lett 86:053102
Wei D, Scherer MR, Bower C, Andrew P, Ryhänen T, Steiner U (2012) A nanostructured electrochromic supercapacitor. Nano Lett 12:1857
Zhu J, Cao L, Wu Y, Gong Y, Liu Z, Hoster HE et al (2013) Building 3D structures of vanadium pentoxide nanosheets and application as electrodes in supercapacitors. Nano Lett 13:5408
Amano F, Tanaka T, Funabiki T (2004) Steady-state photocatalytic epoxidation of propene by O2 over V2O5/SiO2 photocatalysts. Langmuir 20:4236
Eyert V, Höck K-H (1998) Electronic structure of V2O5: role of octahedral deformations. Phys Review B 57:12727
Willinger MG, Pinna N, Su DS, Schlögl R (2004) Geometric and electronic structure of γ−V2O5: comparison between α−V2O5 and γ−V2O5. Phys Review B 69:155114
Jang WL, Lu YM, Chen CL, Lu YR, Dong CL, Hsieh PH et al (2014) Local geometric and electronic structures of gasochromic VOx films. Phys Chem Chem Phys 16:4699
Wei-Luen J, Yang-Ming L, Ying-Rui L, Chi-Liang C, Chung-Li D, Wu-Ching C et al. (2013) Effects of oxygen partial pressure on structural and gasochromic properties of sputtered VOx thin films. Thin Solid Films 544:448
Wong J, Lytle FW, Messmer RP, Maylotte DH (1984) K-edge absorption spectra of selected vanadium compounds. Phys Review B 30:5596
Yamamoto T (2008) Assignment of pre-edge peaks in K-edge x-ray absorption spectra of 3d transition metal compounds: electric dipole or quadrupole? X‐Ray Spectrom 37:572
Marco G, Stefano P, Smyrla WH, Sanjeev M, Yangb XQ, James MB (1999) In situ X‐ray absorption spectroscopy characterization of V2O5 xerogel cathodes upon lithium intercalation. J Electrochem Soc 146:2387
Chaurand P, Rose J, Briois V, Salome M, Proux O, Nassif V et al (2007) New methodological approach for the vanadium K-edge X-ray absorption near-edge structure interpretation: application to the speciation of vanadium in oxide phases from steel slag. J Phys Chem B 111:5101
Stizza S, Mancini G, Benfatto M, Natoli CR, Garcia J, Bianconi A (1989) Structure of oriented V2O5 gel studied by polarized x-ray-absorption spectroscopy at the vanadium K edge. Phys Review B 40:12229
Chen Z, Augustyn V, Wen J, Zhang Y, Shen M, Dunn B et al (2011) High-performance supercapacitors based on intertwined CNT/V2O5 nanowire nanocomposites. Adv Mater 23:791
Acknowledgments
This study was financially supported by the National Science Council of Taiwan under contract nos. MOST 104-2112-M-032-008-MY3 and 102-2112-M-001-004-MY3.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing Interests
The authors declare that they have no competing interests.
Authors’ Contributions
CLD and YRL designed the work and helped to draft the manuscript. TZW and YRL carried out in situ X-ray absorption spectroscopy, SEM, and CV measurements. CLC, JLC, DHW, and WCC supervised this project. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Lu, YR., Wu, TZ., Chen, CL. et al. Mechanism of Electrochemical Deposition and Coloration of Electrochromic V2O5 Nano Thin Films: an In Situ X-Ray Spectroscopy Study. Nanoscale Res Lett 10, 387 (2015). https://doi.org/10.1186/s11671-015-1095-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11671-015-1095-9