Abstract
Based on highly ordered TiO2 nanotube arrays (NTAs), we successfully fabricated the Cu2O-TiO2 NTA heterojunction by a simple thermal decomposition process for the first time. The anodic TiO2 NTAs were functioned as both “nano-container” and “nano-reactors” to load and synthesize the narrow band Cu2O nanoparticles. The loaded Cu2O expanded absorption spectrum of the TiO2 NTAs from ultraviolent range to visible light range. We found that the Cu2O-TiO2 NTA heterojunction films had visible activity towards photocatalytic degrading methyl orange (MO). The photocatalytic abilities of the Cu2O-TiO2 NTA heterojunction films were found increased with the Cu2O content from 0.05 to 0.3 mol/L. This could be explained by more electron-hole pairs generated and less recombination, when the Cu2O-TiO2 heterojunction got formed. Here, we put forward this promising method, hoping it can facilitate the mass production and applications of Cu2O-TiO2 NTA heterojunction.
Similar content being viewed by others
Background
With more and more attention paid to the environmental issues nowadays, the study of water treatment materials emerged in a continuous stream [1,2,3,4]. Hundreds of strategies were proposed for the treatment of polluted water. However, there were many problems, such as low efficiency, low recycling rate, and secondary environment pollution, restricting their further applications [5,6,7]. The semiconductor materials were considered to be a promising candidate, and titanium oxide was recognized as one of the best photocatalyst materials due to its high photocatalytic activity and good chemical and mechanical stability [8,9,10,11,12]. Recently, TiO2 materials with nanotube (NT) array were widely studied, and the tubular morphology was proved to be a promising structure for photocatalysis. Compared with other microcosmic morphologies, TiO2 NT arrays owned several significant advantages [13,14,15,16,17]. Firstly, unique tubular structure could enhance electron-transporting efficiency and restrain the recombination of carriers, which will further produce more reactive oxygen species (ROS) [18, 19]. Secondly, TiO2 NT arrays are much easier to recycle than the TiO2 powder photocatalysts [20,21,22,23,24]. Thirdly, TiO2 NT arrays have large specific surface area and high surface energy. However, due to the relatively wide gaps (~ 3.2 eV). TiO2 NT photocatalyst is only active under UV irradiation [25,26,27,28]. In fact, a photocatalyst that is able to respond with visible light will surely take obvious advantages. At present, the focus of photocatalyst research is to adjust their light response band and improve their photocatalytic efficiency.
Building heterogeneous TiO2 photocatalysts with narrow band gaps is one of the hotspots as an attempt to overcome such impediments. Narrow band semiconductors, like Cu2O, CdS, CdTe, PbS, and Bi2O3, have been studied to build TiO2 heterojunction photocatalysts [29,30,31,32,33,34]. Among them, Cu2O (with the direct gap of ~ 2.2 eV) is regarded as one of the best candidates. For Cu2O, the response band is about 560 nm, and its band gap structure happens to well match with the energy level of TiO2 NTs. As schematically shown in Fig. 1, under the excitation of visible light, electron/hole pairs are generated and the photoinduced electrons are excited to the conduction band of Cu2O and then transfer to the conduction band of TiO2, which suppresses the recombination of electrons and holes. This heterojunction structure solves the problem that the TiO2 materials could not respond to visible light and the problem that electron/hole pairs generated on Cu2O get recombined easily. From this point of view, Cu2O-TiO2 NTA heterojunction structure materials guaranteed a natural advantage in visible light photocatalysis.
General approach to prepare Cu2O-TiO2 heterojunction photocatalysts includes chemical coprecipitation and electrodeposition, and the products have shown promising photocatalytic performances. But it is still a challenge to prepare Cu2O-TiO2 heterojunction photocatalysts with good quality by using a facile and low-cost method. Inspired by the concept of the precursor from Chemical vapor deposition (CVD), the idea, using acetate to carry copper ion to get into the inside of TiO2 NTs prepared by anodic oxidation, comes out. It is known that metal organic compounds are likely to get thermally decomposed. In this study, anodic TiO2 NTAs were functioned as “nano-container” to load copper acetate at first and then as “nano-reactors” to provide space for thermal decomposing the loaded copper acetate. After a thermal treatment, Cu2O-TiO2 TNA heterojunction films were successfully obtained. To the best our knowledge, this method has not been reported to prepare Cu2O-TiO2 TNA heterojunction. Furthermore, the phase composition, morphology, and photocatalytic activity were characterized by XRD, EDS, SEM, and spectrophotometer.
Experimental Section
The chemicals which were mentioned in the experiment process were purchased (Sinopharm Group Chemical Reagent Co. Ltd., China) and used without further purification, except the deionized water with a resistance of 18.3 MΩ cm.
Preparation of Pure TiO2 Nanotube Arrays
Anodic oxidation method was used to prepare uniform and stable TiO2 NTAs with vertical alignment [35, 36]. Metal titanium (Ti) sheets were cut into pieces of 1.5 × 5 cm2 and cleaned by a cleanser. After a sonication bath in ethanol, the Ti pieces were dried in oven. Electrolyte consisted of 535.45 g glycol, 10 g deionized water, and 1.6617 g NH4F, which were mixed and stirred for 2 h. Then, we took two pieces of Ti as anode and cathode, respectively. Immersing them into electrolyte, applying a constant potential of 50 V for 2 h, amorphous TiO2 nanotube arrays (TiO2 NTAs) were fabricated at room temperature.
Synthesis of Cu2O-TiO2 NTA Heterojunction
The amorphous TiO2 NTAs were crystallized into anatase by a thermal treatment at 450 °C. And then, they were used as substrate to prepare Cu2O-TiO2 NTA heterojunction film. First, cupric acetate (Cu(Ac)2) with different concentration was prepared, ranging from 0.05 to 0.3 mol/L. Then, annealed TiO2 NTAs were immersed into the solution transiently and dried in oven at 70 °C immediately. And the final products, Cu2O-TiO2 films, were marked as sample S1-S5 respectively by the different Cu(Ac)2 concentration of 0.05, 0.1, 0.2, 0.3, and 4 mol/L in this immersing process. After this process, the cupric acetate molecules had got into the TiO2 nanotubes. Next step was putting the samples into an atmosphere-sintering furnace of N2 with a sintering temperature of 400 °C for 150 min. The cupric acetate was thermal decomposed in a way described by Eq. (1). Finally, the Cu2O-TiO2 NTA heterojunction films were prepared. This process was schematically shown in Fig. 2.
Just like holding a test tube containing cupric acetate, after heating, cupric acetate thermal decomposed into Cu2O which was left inside the TiO2 NTAs.
Characterization
A scanning electron microscopy (SEM, JSM-7000F, JEOL Inc., Japan) with energy dispersive spectrometer (EDS) was used for the observation of the morphology and structure. The samples were characterized by a D/max-2400 X-ray diffraction spectrometer (Rigaku, D/max-2400, Japan) and a UV-vis spectrometry (Ultrospec 2100 pro) was also used. To evaluate the photocatalytic activity of the as-synthesized Cu2O-TiO2 NTA heterojunction, we took methyl orange (MO), a typical organic indicator, as the degraded object. The Cu2O-TiO2 NTA films (3.0 × 1.5 cm2) were immersed in 5 × 10−5 mol/L of MO aqueous solution and irradiated with seven 4 W visible bulbs (Toshiba, Cool white, FL4W, Japan). Then, the solution was magnetically stirred in the dark for 30 min to ensure adsorption-desorption equilibrium prior to photocatalytic degradation. Photodegradation experiments lasted 180 min with 1.5 mL samples withdrawn periodically. The concentration of the residual MO was measured by a spectrophotometer at about 460 nm on the basis of the Beer-Lambert law. The degradation efficiency of the MO could be defined as follows:
And the varying of At/A0 referred to the changing in Ct, which represented the photocatalytic activity of the tested samples.
Results and Discussion
Figure 3 shows a typical SEM observation of the pure anodic TiO2 NTAs after annealing at 450 °C. Anodizing is an electrolytic process which converts the outer surface of metals into an oxide layer or pore structure. As shown in Fig. 3, the as-prepared TiO2 NTs have open-tube morphology with a uniform outer diameter distribution of ∼ 100 nm. The anodic TiO2 NTAs are highly ordered and oriented, and each single TiO2 NT owns very smooth tube walls with an average thickness of ∼ 10 nm. Our former studies have shown that tube length, the diameter, and morphology could be manipulated by adjusting the anodization protocols [37, 38]. The SEM results also indicate the thermal annealing at a high temperature of 450 °C does not destroy morphologies of the TiO2 NTAs. XRD is used to characterize the crystalline of the pure TiO2 NTAs (sample 1), see Fig. 4a. Results show that diffraction peaks locating at 25.3°, 36.9°, 37.8°, 48°, 53.9°, 55°, 62.7°, and 68.8° could be observed in sample 1, attributing to the (101), (103), (004), (200), (105), (211), (204), and (116) of anatase phase, respectively. As we know, there are three types of titanium dioxide phase, anatase, brookite, and rutile. Rutile could show relatively good photocatalytic ability with a granularity less than 10 nm. However, to get a rutile phase, the TiO2 sample needs to be heated up to a high sinter temperature of 800 °C, which could lead to the break of TiO2 tubes in this case. Brookite phase is hardly to be formed by using thermal annealing method for the bad thermodynamic phase stability, while anatase is the most common phase with good photocatalytic activity [39, 40]. The sharp diffraction peaks and the strong intensity of sample 1 (see Fig. 4a) indicated a highly crystallized anatase structure, which meant our TiO2 substrate was excellent not only in the morphology but also in the crystalline phase. The highly ordered TiO2 NTAs with open-tube mouth morphology were used as substrate to prepare Cu2O-TiO2 NTA heterojunction films in this study.
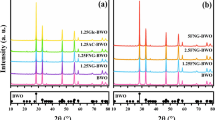
a XRD patterns of the Cu2O-TiO2 NTA heterojunction samples. Sample S0: pure anatase TiO2 NTA sample; samples S2, S4, and S5: thermally decomposed sample with immersing in 0.1, 0.3, and 4 mol/L Cu(Ac)2 solution, respectively. b EDS result of the Cu2O-TiO2 NTA heterojunction films, showing the existence of elements Ti, Cu, and O. The results confirm the successful loading of Cu2O on the TiO2 NTAs
XRD patterns of the TiO2 NTAs loaded with Cu2O nanoparticles in concentration gradient ranging from 0.05 to 4.0 mol/L are also shown in Fig. 4a, and the 4.0 mol/L sample was prepared by a cycling immerse process described in Additional file 1, the “Experimental Details” part. The samples were named samples 2 to 4 with the increasing Cu(Ac)2 concentration. Except the TiO2 peaks, there was no peak of Cu2O showing up in sample 2 because of the tiny amount of the loading Cu2O particles. And particles might be decorated inside of the TiO2 “nano-container” which also raised the difficulty for characterization. In sample 3 and sample 4, obvious cuprite peaks could be observed at 29.6°, 36.4°, 42.3°, and 61.3°, attributing to the cuprite (110), (111), (200), and (220) of Cu2O, respectively. It should be noted here that sample 4 was only used to characterize the existence of Cu2O particles, and its synthetic details were described in Additional file 1. Moreover, the lattice parameters and the grain size were calculated based on the XRD data. After removing the background and Kα2 diffraction, and following the smoothing and fitting process, we got the average lattice parameters of our samples of a = b = c = 4.2646 Å, which matched with the standard PDF. The standard PDF showed that the lattice parameters of Cu2O are: a = b = c = 4.2696 Å, and Cu2O had a cubic structure [41]. The average grain size of Cu2O was calculated as ~ 47 nm, by using Debye-Scherrer formula:
In Eq. (3), D is the grain size, K is the Scherrer constant, γ is the wavelength of X-ray, B is FWHM which needs to be in the radian, and θ is the diffraction angle. XRD results indicate that the Cu(Ac)2 were loaded into the TiO2 NTAs and successfully decomposed into Cu2O inside the same TiO2 NTAs, and then the Cu2O-TiO2 NTA heterojunction films got formed. To further investigate the Cu2O-TiO2 NTA heterojunction, an elemental analysis was carried out by using EDS. Figure 4b showed an EDS diagram of Cu2O-TiO2 NTA heterojunction film which was prepared with 0.2 mol/L Cu(Ac)2. The atomic percentages were 7.32, 28.96, 57.45, and 6.27% for elements Cu, Ti, O, and impurity C. This result showed that the Cu2O owned a relatively low content in the heterojunction sample, but it still brought about the visible-light activity, which would be discussed later in the MO degradation experiment. The EDS results well agreed with the XRD results in Fig. 4a that cuprite Cu2O was successfully loaded to anatase NTAs.
Figure 5 showed the top view SEM results of the modified TiO2 NTAs. Compared with the pure TiO2 NTA samples in Fig. 3, a few small particles could be seen near the upper and inside of the TiO2 tubes in Fig. 5a. Increasing the modification amount, a number of nanoparticles could be observed obviously in Fig. 5b. Figure 5c was sample 4 that we discussed before. Large parts of the tube surface were covered by the redundant Cu2O, indicating that sample 4 was over-decorated. Based on the SEM images, the size distribution of Cu2O particles was estimated ranging from ~ 30 to ~ 80 nm, which well agreed with XRD calculated grain size of ~ 47 nm. For the tubular structure of the three samples, they still retained the vertical alignment state, but some tubes got a little awry. It was considered as the influence of thermal decomposition process, which needed a heating process of 400 °C to get Cu(Ac)2 decomposed into Cu2O. High temperature in the decomposition step had a negative effect on the tubular structure, supported by the SEM images. However, if the heating temperature in the thermal process went too low to 240 °C, Cu(CH3COO)2·H2O would just get dehydrated instead of decomposed. So the temperature should be controlled in ~ 300 to 400 °C to keep the nano-scale tubular structure and ensure the fabrication of Cu2O-TiO2 NTA heterojunction. It can be concluded that the Cu2O-TiO2 heterojunction could be formed, and the morphology retained well, when the decomposition happens at 400 °C.
The Cu2O nanoparticles were loaded on TiO2 NTAs to fabricate the heterojunction, which was expected to enhance the photo-response ability in visible light range, so UV-vis characterization was adopted to investigate optical properties of the as-synthesized Cu2O-TiO2 NTAs. Figure 6a shows the UV-vis absorption spectra of the Cu2O-TiO2 NTA samples with Cu2O-loaded magnitude increasing from none to 4.0 mol/L. It could be seen in Fig. 6a that the pure TiO2 NTAs without loading Cu2O only showed out a high absorption in the ultraviolet region (< 380 nm), due to its intrinsic material properties. After loading the Cu2O particles, the absorption range was expanded to 600–700 nm. And when the intensity is increasing with the raising of the Cu2O modification magnitude, the absorption value of the Cu2O-TiO2 heterojunction films also got increased. Figure 6a indicated that TiO2 NTAs were given the visible light response ability by decorating Cu2O nanoparticles. UV-vis along with SEM, EDS, and XRD results proved that the Cu2O-TiO2 NTA heterojunction was fabricated successfully by the thermal decomposition method, and samples showed the enhanced visible light absorption.
a UV-vis spectra of the Cu2O-TiO2 NTAs and absorption get expanded to visible light range and raised with the loading amount of Cu2O. b Visible-light photocatalytic degradation kinetics of MO treated by the heterojunction films with different Cu2O content. When decoration magnitude of Cu2O on TiO2 NTAs increased, the MO decomposition efficiency under visible light irradiation raised. Sample S0 referred to pure TiO2 film, and sample S1–S5 were the samples immersed in Cu(Ac)2 solution with the concentrations of 0.05, 0.1, 0.2, 0.3, and 4 mol/L, respectively
Photocatalytic activities, one of the most important properties of the Cu2O-TiO2 NTA films, were evaluated through degradation of MO aqueous solution. The visible-light photocatalytic degradation kinetics was shown in Fig. 6b. The MO degradation rate was proportion to the loading amount of Cu2O approximately. The more Cu2O particles were loaded on TiO2 NTAs, the faster MO got degraded. Sample S1 degraded MO to 91.0% in 3 h under visible light irradiation, while sample S4 degraded MO to 86.4% in 3 h under the same condition. MO degradation rate represented the photocatalytic activity of the samples. Comparing with the photocatalytic degradation rate to the ~ 2.73% of CdTe-TiO2 by a pulse electrodeposited method [29], ~ 45% of Bi2O3 by a ultrasonication-assisted successive ionic layer adsorption and reaction (SILAR) technique [32], and ~ 27.25% of Cu2O by a square wave voltammetry method [33], photoactivity of this as-synthesized Cu2O-TiO2 sample was improvable. However, as a facile new strategy, it still conduced to improve fabrication method. When the Cu2O loading amount went up, there was a trend that the photocatalytic activity of our as-synthesized Cu2O-TiO2 NTA heterojunction films increased. It indicated the Cu2O content had a positive influence of the visible-light photocatalytic activity. TiO2 itself only responded to ultraviolet, and the visible light range photocatalytic ability should come from the decoration of Cu2O. As shown in Fig. 7, the conduction band bottom of Cu2O was a little higher than that of TiO2, while the valence band top of Cu2O was higher than that of TiO2. So, the photoinduced electrons were excited to the conduction band of Cu2O and then transferred to the conduction band of TiO2. As a direct-gap semiconductor, wave vector of Cu2O was just the same at the bottom of the conduction band and the top of valence band. It meant that only the changes of energy were required, instead of the changes of momentum. This energy band structure led to the situation that carriers recombined easily. However, due to the help of heterojunction structure, the photogenerated electrons on Cu2O transferred to TiO2 NTAs which suppressed the recombination of electron/hole pairs. The longer the pairs existed, the more easily the ROS got produced which brought this photocatalytic activity. As more Cu2O loaded on TiO2 NTAs, the heterojunction fabricated better. And the photocatalytic ability got promoted. So, the Cu2O content showed a positive influence of the visible-light photocatalytic activity. However, further increase of Cu2O content as well as the photocatalytic ability is limited, due to the solubility of Cu(Ac)2 in aqueous solution which was 7.2 g (0.36 mol/L) at room temperature. And sample S5 with Cu(Ac)2 concentration of 4.0 mol/L is prepared by a cycling immerse process described in Additional file 1, the Experimental Details part. The photocatalytic degradation of the MO followed pseudo-first-order kinetics [42] and the kinetic reaction could be expressed as:
While our degradation curve showed nearly a straight line, it is not an exponential function. So, there was still room for improvement. And the solubility limit could be broken by the repeated immersion method we mentioned before, with further investigation of the Cu(Ac)2 concentration and repetition times to avoid adverse effects. In this study, as this thermal decomposition method was what we concerned and tried to illustrate, we just took the 0.3 mol/L (close the solubility of 0.36 mol/L) as the maximum concentration of Cu(Ac)2 solution. And the photocatalytic activity in visible light range of our as-synthesized heterojunction was confirmed by the MO degradation results. Our previous study found that the Degussa P25 had similar ultraviolet photocatalytic activities with TiO2 NTAs, when the power P25 was placed on a glass substrate [28]. It can be concluded that we have successfully prepared Cu2O-TiO2 NTA heterojunction films with visible-light photocatalytic activities.
Conclusions
In summary, we have successfully prepared the Cu2O-TiO2 NTA heterojunction films by a simple thermal decomposition process. SEM, EDS, and XRD results show that TiO2 NTAs with a tube diameter of ~ 100 nm were loaded by Cu2O nanoparticles with an average size of ~ 50 nm. The anodic TiO2 NTAs functioned as both “nano-container” and “nano-reactors” to load and synthesize the narrow-band Cu2O nanoparticles, which has not reported before. UV-vis spectra indicate that the absorption range of the TiO2 NTAs was expanded from ultraviolent range to visible light range, due to the loading of Cu2O. Photocatalytic testing indicated that there was a visible-light photocatalytic activity of the as-synthesized Cu2O-TiO2 heterojunction. The photocatalytic abilities of the Cu2O-TiO2 NTA heterojunction films were found to be increased with the Cu2O content from 0.05 to 0.3 mol/L. Our current work has demonstrated a novel and facile method to prepare Cu2O-TiO2 NTA heterojunction films, which could also be promising for environmental and energy-related areas.
Abbreviations
- EDS:
-
Energy-dispersive spectrometry
- NTAs:
-
Nanotube arrays
- SEM:
-
Scanning electron microscopy
- XRD:
-
X-ray diffraction
References
Qu X, Brame J, Li Q, Alvarez PJJ (2013) Nanotechnology for a safe and sustainable water supply: enabling integrated water treatment and reuse. Acc Chem Res 46:834–843
Lim YW, Tang Y, Cheng YH, Chen Z (2010) Morphology, crystal structure and adsorption performance of hydrothermally synthesized titania and titanate nanostructures. Nanoscale 2:2751–2757
Choudhury B, Borah B, Choudhury A (2012) Extending photocatalytic activity of TiO2 nanoparticles to visible region of illumination by doping of cerium. Photochem Photobiol 88:257–264
Dholam R, Patel N, Adami M, Miotello A (2009) Hydrogen production by photocatalytic water-splitting using Cr- or Fe-doped TiO2 composite thin films photocatalyst. Int J Hydrog Energy 34:5337–5346
Brame JA, Hong SW, Lee J, Lee SH, Alvarez PJ (2013) Photocatalytic pre-treatment with food-grade TiO2 increases the bioavailability and bioremediation potential of weathered oil from the Deepwater Horizon oil spill in the Gulf of Mexico. Chemosphere 90:2315–2319
Jiang Z, Tang Y, Tay Q, Zhang Y, Malyi OI, Wang D, Deng J, Lai Y, Zhou H, Chen X, Dong Z, Chen Z (2013) Understanding the role of nanostructures for efficient hydrogen generation on immobilized photocatalysts. Adv Energy Mater 3:1368–1380
Fischer BB, Krieger-Liszkay A, Eggen RIL (2004) Photosensitizers neutral red (type I) and rose bengal (type II) cause light-dependent toxicity in Chlamydomonas reinhardtii and induce the Gpxh gene via increased singlet oxygen formation. Environ Sci Technol 38:6307–6313
Thelese Ru Bao F, Chan KL, Hu X (2012) Structure and properties of nano-confined poly(3-hexylthiophene) in nano-array/polymer hybrid ordered-bulk heterojunction solar cells. Nanoscale 4:478–485
Foong TRB, Shen Y, Hu X, Sellinger A (2010) Template-directed liquid ALD growth of TiO2 nanotube arrays: properties and potential in photovoltaic devices. Adv Funct Mater 20:1390–1396
Jha PK, Gupta SK, Lukacevic I (2013) Electronic structure, photocatalytic properties and phonon dispersions of X-doped (X = N, B and Pt) rutile TiO2 from density functional theory. Solid State Sci 22:8–15
Mankad V, Gupta SK, Jha PK (2011) Low frequency Raman scattering of anatase titanium dioxide nanocrystals. Physica E-Low-Dimensional Syst Nanostruct 44:614–617
Mohapatra SK, Misra M, Mahajan VK, Raja KS (2007) Design of a highly efficient photoelectrolytic cell for hydrogen generation by water splitting: application of TiO2-xCx nanotubes as a photoanode and Pt/TiO2 nanotubes as a cathode. J Phys Chem C 111:8677–8685
Gong D, Grimes CA, Varghese OK, Hu WC, Singh RS, Chen Z, Dickey EC (2001) Titanium oxide nanotube arrays prepared by anodic oxidation. J Mater Res 16:3331–3334
Grimes CA (2007) Synthesis and application of highly ordered arrays of TiO2 nanotubes. J Mater Chem 17:1451–1457
Lai Y, Gao X, Zhuang H, Huang J, Lin C, Jiang L (2009) Designing superhydrophobic porous nanostructures with tunable water adhesion. Adv Mater 21:3799–3803
Macak JM, Schmuki P (2006) Anodic growth of self-organized anodic TiO2 nanotubes in viscous electrolytes. Electrochim Acta 52:1258–1264
Roy P, Berger S, Schmuki P (2011) TiO2 nanotubes: synthesis and applications. Angewandte Chemie-Int Ed 50:2904–2939
Khan MA, Akhtar MS, Woo SI, Yang OB (2008) Enhanced photoresponse under visible light in Pt ionized TiO2 nanotube for the photocatalytic splitting of water. Catal Commun 10:1–5
Macak JM, Zlamal M, Krysa J, Schmuki P (2007) Self-organized TiO2 nanotube layers as highly efficient photocatalysts. Small 3:300–304
Mishra S, Jha PK, Pratap A (2012) Study of size-dependent glass transition and Kauzmann temperature of titanium dioxide nanoparticles. J Therm Anal Calorim 107:65–68
Wang P, Tang Y, Dong Z, Chen Z, Lim T-T (2013b) Ag-AgBr/TiO2/RGO nanocomposite for visible-light photocatalytic degradation of penicillin G. J Mater Chem A 1:4718–4727
Wang S, Qian H, Hu Y, Dai W, Zhong Y, Chen J, Hu X (2013a) Facile one-pot synthesis of uniform TiO2-Ag hybrid hollow spheres with enhanced photocatalytic activity. Dalton Trans 42:1122–1128
Xiu Z-m, Zhang Q-b, Puppala HL, Colvin VL, Alvarez PJJ (2012) Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Lett 12:4271–4275
Choudhury B, Choudhury A (2013) Oxygen vacancy and dopant concentration dependent magnetic properties of Mn doped TiO2 nanoparticle. Curr Appl Phys 13:1025–1031
Park JH, Kim S, Bard AJ (2006) Novel carbon-doped TiO2 nanotube arrays with high aspect ratios for efficient solar water splitting. Nano Lett 6:24–28
Woan K, Pyrgiotakis G, Sigmund W (2009) Photocatalytic carbon-nanotube-TiO2 composites. Adv Mater 21:2233–2239
Zheng Q, Zhou B, Bai J, Li L, Jin Z, Zhang J, Li J, Liu Y, Cai W, Zhu X (2008) Self-organized TiO2 nanotube array sensor for the determination of chemical oxygen demand. Adv Mater 20:1044–1049
Liao Y, Brame J, Que W, Xiu Z, Xie H, Li Q, Fabian M, Alvarez PJ (2013a) Photocatalytic generation of multiple ROS types using low-temperature crystallized anodic TiO2 nanotube arrays. J Hazard Mater 260:434–441
Feng H, Tran TT, Chen L, Yuan L, Cai Q (2013) Visible light-induced efficiently oxidative decomposition of p-nitrophenol by CdTe/TiO2 nanotube arrays. Chem Eng J 215:591–599
Yang H, Fan W, Vaneski A, Susha AS, Teoh WY, Rogach AL (2012) Heterojunction engineering of CdTe and CdSe quantum dots on TiO2 nanotube arrays: intricate effects of size-dependency and interfacial contact on photoconversion efficiencies. Adv Funct Mater 22:2821–2829
Cai F, Yang F, Zhang Y, Ke C, Cheng C, Zhao Y, Yan G (2014) PbS sensitized TiO2 nanotube arrays with different sizes and filling degrees for enhancing photoelectrochemical properties. Phys Chem Chem Phys 16:23967–23974
Ge M, Cao C, Li S, Zhang S, Deng S, Huang J, Li Q, Zhang K, Al-Deyab SS, Lai Y (2015) Enhanced photocatalytic performances of n-TiO2 nanotubes by uniform creation of p-n heterojunctions with p-Bi2O3 quantum dots. Nanoscale 7:11552–11560
Zhang J, Wang Y, Yu C, Shu X, Jiang L, Cui J, Chen Z, Xie T, Wu Y (2014) Enhanced visible-light photoelectrochemical behaviour of heterojunction composite with Cu2O nanoparticles-decorated TiO2 nanotube arrays. New J Chem 38:4975–4984
Mazare A, Liu N, Lee K, Killian MS, Schmuki P (2013) Embedded palladium activation as a facile method for TiO2-nanotube nanoparticle decoration: Cu2O-induced visible-light photoactivity. Chemistryopen 2:21–24
Li H, Lai Y, Huang J, Tang Y, Yang L, Chen Z, Zhang K, Wang X, Tan LP (2015) Multifunctional wettability patterns prepared by laser processing on superhydrophobic TiO2 nanostructured surfaces. J Mater Chem B 3:342–347
Seong WM, Kim DH, Park IJ, Do Park G, Kang K, Lee S, Hong KS (2015) Roughness of Ti substrates for control of the preferred orientation of TiO2 nanotube arrays as a new orientation factor. J Phys Chem C 119:13297–13305
Zhong P, Que W, Liao Y, Zhang J, Hu X (2012) Improved performance in dye-sensitized solar cells by rationally tailoring anodic TiO2 nanotube length. J Alloys Compd 540:159–164
Liao YL, Zhang HW, Que WX, Zhong P, Bai FM, Zhong ZY, Wen QY, Chen WH (2013b) Activating the single-crystal TiO2 nanoparticle film with exposed {001} facets. ACS Appl Mater Interfaces 5:6463–6466
Hanaor DAH, Sorrell CC (2011) Review of the anatase to rutile phase transformation. J Mater Sci 46:855–874
Kandiel TA, Robben L, Alkaim A, Bahnemann D (2013) Brookite versus anatase TiO2 photocatalysts: phase transformations and photocatalytic activities. Photochem Photobiol Sci 12:602–609
Brandt IS, Martins CA, Zoldan VC, Viegas ADC, da Silva JHD, Pasa AA (2014) Structural and optical properties of Cu2O crystalline electrodeposited films. Thin Solid Films 562:144–151
Liao Y, Zhang H, Zhong Z, Jia L, Bai F, Li J, Zhong P, Chen H, Zhang J (2013c) Enhanced visible-photocatalytic activity of anodic TiO2 nanotubes film via decoration with CuInSe2 nanocrystals. ACS Appl Mater Interfaces 5:11022–11028
Funding
This work was financially supported by the National R&D Program of China under Nos.2017YFA0207400 and 2016YFA0300801, National Natural Science Foundation of China under Nos.51502033, 61734002, and 61571079, and Fundamental Research Funds for the Central Universities of China, No.2672018ZYGX2018J030.
Availability of Data and Materials
They are all in the main text and figures.
Author information
Authors and Affiliations
Contributions
YL conceived and supervised the research. PD and XW conducted the experiments and wrote the manuscript. DZ, FL, QY, and HZ made the theoretical analysis. ZZ checked this article and put forward some revised opinions. All the authors discussed the results. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
The experimental details of preparing the Cu2O-TiO2 samples and further characterization results of Raman spectra and XRD patterns are provided as the supplemental information to support the discussion. (DOCX 2387 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Liao, Y., Deng, P., Wang, X. et al. A Facile Method for Preparation of Cu2O-TiO2 NTA Heterojunction with Visible-Photocatalytic Activity. Nanoscale Res Lett 13, 221 (2018). https://doi.org/10.1186/s11671-018-2637-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11671-018-2637-8