Abstract
Surface chemistry involved in the epitaxy of graphene by sublimating Si atoms from the surface of epitaxial 3C-SiC(111) thin films on Si(111) has been studied. The change in the surface composition during graphene epitaxy is monitored by in situ temperature-programmed desorption spectroscopy using deuterium as a probe (D2-TPD) and complementarily by ex situ Raman and C1s core-level spectroscopies. The surface of the 3C-SiC(111)/Si(111) is Si-terminated before the graphitization, and it becomes C-terminated via the formation of C-rich (6√3 × 6√3)R30° reconstruction as the graphitization proceeds, in a similar manner as the epitaxy of graphene on Si-terminated 6H-SiC(0001) proceeds.
Similar content being viewed by others
Introduction
Graphene, a single layer of sp2-bonded carbon, has fabulous electronic, mechanical, and optical properties [1]. Graphene is thus expected to be various kinds of applications. Owing to its industrial adaptability, epitaxial graphene (EG) formed by annealing of SiC bulk crystal is attracting recent attentions [2–4]. EG, however, face two challenges: the limited diameter of the substrate and the low cost-performance in the current price. To overcome these challenges, we have been investigating the use of SiC thin films on Si substrates, instead of SiC bulk crystals, in the formation of graphene. We have actually succeeded in fabricating a graphene on an epitaxial SiC thin film on Si substrate by sublimating silicon atoms from the surface of the epitaxial SiC thin film [5, 6]. This graphene-on-silicon (GOS) method has a potential of forming graphene films on large-scale Si wafers. GOS is therefore compatible with the silicon technology [5, 6].
The film quality of GOS, however, still remains as a challenge. This undoubtedly requires detailed understanding of the graphitization mechanism in GOS as a prerequisite. In the case of epitaxial graphene formation on Si-face 4H- or 6H-SiC(0001) substrates, several groups [2–4] have reported that graphene (1 × 1) is formed after the appearance of two SiC reconstructions: (√3 × √3)R30° and (6√3 × 6√3)R30° in this order. GOS process on Si(111) substrates follows this process [7]. To go further into the clarification of the mechanism of graphitization, however, the surface chemical component for each reconstruction needs to be probed. Hirayama et al. conducted temperature-programmed desorption measurement on molecular hydrogen (H2-TPD) for each of the reconstructed surfaces of 6H-SiC(0001) [8]. They concluded that the hydrogen adsorption site shifts from Si to C after graphitization, but detailed identification of the H2-TPD peaks has not been given yet. In this study, we have conducted TPD using molecular deuterium (D2-TPD) to investigate the surface chemistry involved in the GOS process on 3C-SiC(111)/Si(111). TPD serves as an in situ probe for the chemical component of the surface. C1s core-level and Raman spectroscopies have also been used as complementary means to support the understandings given by D2-TPD.
Experiment
A p-type Si(111) wafer (0.400–0.600 Ω cm) was cut into pieces to form specimens sized with 7 × 40 mm2. The specimen, after degreased by ultrasonication in acetone and ethanol, was introduced into the UHV chamber (base pressure: ~10−10 torr) and flash-annealed at 1,473 K (Sample A). The epitaxial 3C-SiC thin films, ~100 nm in thickness, were grown by exposing the DC-heated Si substrate (1,323 K) to a CH3SiH3 (MMS) gas [5, 6]. The samples were then annealed either at 1,273 K for 10 s (Sample B), 1,423 K for 60 min (Sample C), or 1,523 K for 30 min (Sample D) [2, 3]. After annealing, each sample was exposed to a flux of atomic deuterium, made by cracking of D2 molecules (~10−4 Pa) by a hot tungsten filament (1,673 K). D2-TPD spectrum was then obtained by gradually increasing the sample temperature at a rate of 5 K/s. Raman and C1s core-level spectroscopies were also performed ex situ. The excitation energy in the Raman spectroscopy is 2.41 eV. The X-ray source for the C1s core-level spectroscopy is non-monochromatized Mg–Kα (1,253.6 eV).
Results and Discussion
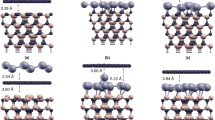
Figure 1 compares the D2-TPD spectra for Si (Sample A), (√3 × √3)R30°-reconstructed 3C-SiC(111)/Si(111) (Sample B), (6√3 × 6√3)R30°-reconstructed 3C-SiC(111)/Si(111) (Sample C), and (1 × 1) graphene/3C-SiC(111)/Si(111) (Sample D) surfaces [7]. The spectrum for Si(111) (Sample A) has two peaks at ~660 and ~780 K, which are ascribable to the deuterium desorption from D2-Si and D-Si, respectively [9]. The dominance of the latter peak indicates that the surface of the Si(111) substrate is largely terminated with monodeuteride. In the spectrum of the (√3 × √3)R30°-reconstructed 3C-SiC(111)/Si(111) surface (Sample B), a new predominant peak appears at ~960 K. This peak is attributable to the deuterium desorption from D-Si backbonded with carbon atoms (D-Si(C)) [10]. Concomitant with the appearance of the predominant peak of D-Si backbonded with carbon atoms, the peaks of D2-Si (660 K) and D-Si (780 K) backbonded with silicon atoms decrease. The absence of peaks in the region ranging from 1,100 to 1,300 K indicates that no carbon atoms are present in the topmost layer. This is in good agreement with the Raman spectrum in Fig. 2(i), showing no graphene-related peaks. The surface of the (√3 × √3)R30°-reconstructed 3C-SiC(111)/Si(111) is thus proven to be Si-terminated.
On the (6√3 × 6√3)R30°-reconstructed 3C-SiC(111)/Si(111) (Sample C), the TPD spectrum consists mainly of D x -Si(C). Here, x = 1 stands for the peak at 960 K and x = 2 for the peak at 850 K [10]. The peaks due to D x -Si (500–800 K) almost disappear. It has been demonstrated in a previous report that the adsorption of hydrogen atoms onto the (6√3 × 6√3)R30°-reconstructed SiC surface breaks the bondings between the reconstructed layer and the SiC interface, so that the surface carbon atoms in the layer are converted into graphene layer, and the SiC interface is terminated with hydrogen [11, 12]. The main peak at 960 K is thus attributable to the deuterium desorption from D-terminated SiC interface that results from the breakage of the bonds between the (6√3 × 6√3)R30°-reconstructed layer and the underneath SiC surface. Small features appear in the temperature range of 1,100–1,400 K. They are due to desorption of deuterium atoms bonded on sp2-bonded carbon atoms by D-exposure [13, 14]. The appearance of these peaks is in agreement with the presence of the (6√3 × 6√3)R30°-reconstructed layer because the reconstructed layer mainly consists of sp2-bonded atoms, and part of the carbon atoms are covalently bonded with the SiC surface [15]. This D2-TPD result is in good agreement with the Raman spectrum of the reconstructed layer. The appearance of the broad G band (~1,600 cm−1) indicates the formation of the cluster of sp2-bonded carbon atoms, i.e., nanographene. The appearance of the D band (~1,350 cm−1) and the absence of the G′ band (~2,700 cm−1), however, indicate a low degree of the crystallinity of the sp2-bonded network of the carbon atoms. C1s core-level spectrum of the (6√3 × 6√3)R30°-reconstructed layer (Fig. 3(i)) actually supports the idea. In the core-level spectrum, the surface components S1 (~285.2 eV) and S2 (~284.0 eV) appear as well as the component due to the bulk of the SiC thin film (~283.0 eV). S1 and S2 are due to the carbon atoms connected with the SiC surface and the sp2-bonded carbon atoms, respectively, in the (6√3 × 6√3)R30°-reconstructed layer [15]. The intensity ratio of S1 and S2 peak is 2, as that of the spectrum of (6√3 × 6√3)R30°-reconstructed layer on 6H-SiC(0001). Thus, the 3C-SiC(111)/Si(111) surface is proven to become C rich and (6√3 × 6√3)R30° reconstructed by annealing the Si-terminated (√3 × √3)R30°-reconstructed surface [15]. This change in the surface chemistry of 3C-SiC(111)/Si(111) is quite similar to that of 6H-SiC(0001) [3, 4, 15].
The TPD spectrum of the graphene overlayer on 3C-SiC(111)/Si(111) (Sample D) drastically changes, as demonstrated in Fig. 1(d). Two distinct peaks around 1,100 K and 1,300 K are due to desorption of deuterium bonded with sp2-bonded carbon atoms in the graphene overlayer, while the peaks due to deuterium desorption from D-Si of the SiC thin film and Si substrate (<1,000 K) are not observed. The Raman spectrum of the graphene overlayer (Fig. 2(iii)) displays the G′ band, in addition to the G and the D bands. The presence of the G′ band indicates the well-ordered graphene overlayer because the appearance of G′ band is the consequence of the high degree of the crystallinity of the graphene layer [16]. Further, the G′ band consists of multiple components, as can be seen from the line shape of the G′ band. This indicates that the graphene layers are Bernal stacked [16]. This is supported by the C1s core-level spectrum (Fig. 3). In the spectrum, the peak due to sp2-bonded carbon atoms (~284.3 eV) is dominant, and the component due to the (6√3 × 6√3)R30°-reconstructed layer (~285.2 eV) is still observed. It can be thus concluded that the graphene overlayer grows on the (6√3 × 6√3)R30°-reconstructed layer as the epitaxy of graphene on 6H-SiC(0001). This can explain the disappearance of the peaks (D x -Si(C)) that is related with the (6√3 × 6√3)R30°-reconstructed layer in the TPD spectrum of sample D, because graphene overlayer blocks adsorption or desorption of deuterium onto the surface.
Conclusion
We have probed the epitaxial processes of graphene on 3C-SiC(111)/Si(111) in situ by D2-TPD spectroscopy, and complemental ex situ spectroscopies, such as Raman spectroscopy and C1s core-level spectroscopy. The results obtained in this study indicate that the epitaxy of graphene on 3C-SiC(111)/Si(111) proceeds in a similar manner to that on hexagonal SiC(0001) bulk crystals.
References
Geim AK: Science. 2009, 324: 1530. COI number [1:CAS:528:DC%2BD1MXnsFOrsLk%3D]; Bibcode number [2009Sci...324.1530G] 10.1126/science.1158877
Riedl C, Starke U, Bernhardt J, Franke M, Heinz K: Phys. Rev. B. 2007, 76: 245406. Bibcode number [2007PhRvB..76x5406R] Bibcode number [2007PhRvB..76x5406R] 10.1103/PhysRevB.76.245406
Rollings E, Gweon G-H, Zhou SY, Mun BS, McChesney JL, Hussain BS, Fedorov AV, First PN, de Heer WA, Lanzara A: J. Phys Chem Solids. 2006, 67: 2172–2177. COI number [1:CAS:528:DC%2BD28XptlSqu7w%3D]; Bibcode number [2006JPCS...67.2172R] 10.1016/j.jpcs.2006.05.010
Mallet P, Varchon F, Naud C, Magaud L, Berger C, Veuillen J-Y: Phys. Rev. B. 2007, 76: 041403. Bibcode number [2007PhRvB..76d1403M] Bibcode number [2007PhRvB..76d1403M] 10.1103/PhysRevB.76.041403
Miyamoto Y, Handa H, Saito E, Konno A, Suemitsu M, Fukidome H, Ito T, Yasui K, Nakazawa H, Endoh T: e-J. Surf. Sci Nanotech.. 2009, 7: 107–109. COI number [1:CAS:528:DC%2BD1MXislyjur8%3D] 10.1380/ejssnt.2009.107
Suemitsu Yu Miyamoto M, Handa Hiroyuki, Konno Atsushi: e-J. Surf. Sci. Nanotech. 2009, 7: 311–313. 10.1380/ejssnt.2009.311
Suemitsu M, Fukidome H: J. Phys. D., in press.
Aoki Y, Hirayama H: Appl. Phys. Lett.. 2009, 95: 094103. Bibcode number [2009ApPhL..95i4103A] Bibcode number [2009ApPhL..95i4103A] 10.1063/1.3223598
Kim H, Taylor N, Spila T, Glass G, Park SY, Greene JE, Abelson JR: Surf. Sci.. 1997, 380: 496–500. 10.1016/S0039-6028(96)01587-7
Konno A, Senthil K, Murata T, Suemitsu M: Appl. Surf. Sci.. 2006, 252: 3692–3696. COI number [1:CAS:528:DC%2BD28Xhs1Gkt7g%3D]; Bibcode number [2006ApSS..252.3692K] 10.1016/j.apsusc.2005.05.052
Riedl C, Coletti C, Iwasaki T, Zakharov AA, Starke U: Phys. Rev. Lett.. 2009, 103: 246804. COI number [1:STN:280:DC%2BC3c3jslGktQ%3D%3D]; Bibcode number [2009PhRvL.103x6804R] 10.1103/PhysRevLett.103.246804
Virojanadara C, Zakharov AA, Yakimova R, Johanson LI: Surf. Sci.. 2007, 604: 4–7. 10.1016/j.susc.2009.11.011
Schenk A, Winter B, Biener J, Lutterioh C, Schubert UA, Küppers J: J. Appl. Phys.. 1995, 77: 6.
Zhao X, Outlaw RA, Wang JJ, Zhu MY, Smith GD, Holloway BC: J. Chem. Phys.. 2006, 124: 194704. COI number [1:STN:280:DC%2BD28zgtlCgsg%3D%3D]; Bibcode number [2006JChPh.124s4704Z] 10.1063/1.2187969
Emtsev KV, Speck E, Seyller Th, Ley L: Phys. Rev. B.. 2008, 77: 155303. Bibcode number [2008PhRvB..77o5303E] Bibcode number [2008PhRvB..77o5303E] 10.1103/PhysRevB.77.155303
Pimenta MA, Dresselhaus G, Dresselhaus MS, Cançado LG, Jorio A, Saito R: Phys. Chem. Chem. Phys.. 2007, 9: 1276. COI number [1:CAS:528:DC%2BD2sXisFCntrc%3D] 10.1039/b613962k
Acknowledgments
The work was supported by CREST, the Japan Science and Technology Agency (JST), Japan.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Abe, S., Handa, H., Takahashi, R. et al. Surface Chemistry Involved in Epitaxy of Graphene on 3C-SiC(111)/Si(111). Nanoscale Res Lett 5, 1888 (2010). https://doi.org/10.1007/s11671-010-9731-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11671-010-9731-x