Abstract
In order to prominently investigate the effects of the surface spin on the magnetic properties, the weak magnetic ZnLa0.02Fe1.98O4 nanoparticles were chosen as studying objects which benefit to reduce as possibly the effects of interparticle dipolar interaction and crystalline anisotropy energies. By annealing the undiluted and diluted ZnLa0.02Fe1.98O4 nanoparticles at different temperatures, we observed the rich variations of magnetic ordering states (superparamagnetism, weak ferromagnetism, and paramagnetism). The magnetic properties can be well understood by considering the effects of the surface spin of the magnetic nanoparticles. Our results indicate that in the nano-sized magnets with weak magnetism, the surface spin plays a crucial rule in the magnetic properties.
Similar content being viewed by others
Background
Ferrite nanocrystals have been extensively studied due to their tunable and remarkable magnetic properties as well as catalytic properties not existing in the corresponding bulk materials [1–5]. In the fundamental research field, magnetic nanoparticles (NPs) usually serve as ideal model systems, e.g., the Stoner-Wohlfarth [6, 7] and Néel-Brown model [8], or to study the finite-size effect [9]. As the size of a magnetic particle decreases, the significance of the surface spins increases, resulting in the various magnetic ordering states such as spin-glass or cluster-glass-like behavior [10–13] or weak ferromagnetism [14, 15] of the surface spins.
Compared with strong magnetic materials which have higher H c and M values, the material with weak magnetism (small values of coercivity H c and magnetization M) is a good candidate for studying the effects of surface spins on magnetic properties, because the strong anisotropy or interparticle dipolar interaction in the strong magnetic system can suppress the effects of surface spin [16, 17]. ZnFe2O4 crystallizes in the bulk in the normal spinel structure with Fe3+ ions (5 μ B moment per Fe3+ ion) occupying octahedral sites and Zn2+ ions (with zero moment) occupying tetrahedral sites. Superexchange interaction between two Fe3+ ions will have their moments aligned anti-parallel to each other which results in the antiferromagnetic (AFM) ZnFe2O4 [18, 19] with the theoretical moment being 0 μ B /f.u. For the La3+ substituted bulk ZnLa0.02Fe1.98O4 (ZLFO), the theoretical moment is 0.1 μ B /f.u., which exhibits the weak ferromagnetism. In the present work, we take ZLFO as a studying object to investigate the effects of surface spins on the magnetic properties. We first prepared ZLFO NPs via a hydrothermal method. Then, some of the NPs were diluted in the Al2O3 matrix and others were undiluted, both followed by annealing at different temperatures of 700°C, 800°C, 900°C, and 1,000°C. The magnetic measurements show that the ZLFO NPs have the weak magnetism with small M and H c values. Our results indicate that the surface spins significantly affect the macromagnetism of ZLFO NPs.
Experimental details
Material preparation
All raw materials include: iron (III) nitrate hexahydrate [Fe(NO3)3 · 6H2O, 99%], zinc nitrate hexahydrate [Zn(NO3)2 · 6H2O, 99%], lanthanum (III) acetate sesquihydrate [La(OOCCH3)3 · 1.5H2O, 99.9%], and aluminum nitrate nonahydrate [Al(NO3)3 · 9H2O], serving as the sources of metallic ions in ZLFO and Al2O3; sodium acetate trihydrate (C2H3NaO2 · 3H2O, 99%) and 1-hexadecyltrimethylammonium bromide (C19H42BrN, 99%), being used as surfactants for improving precursor’s dispersibility; ethylene glycol (C2H6O2, 99%), acting as the solvent.
Firstly, 36 mmol Zn(NO3)2 · 6H2O, 72 mmol Fe(NO3)3 · 6H2O, 7.2 mmol La(OOCCH3)3 · 1.5H2O, and 108 mmol C2H3NaO2 · 3H2O were dissolved in 300-mL anhydrous C2H6O2 with magnetic stirring. Then, 1.08 mmol C19H42BrN was added into the solution with continuous stirring at 40°C for 30 min to get a homogeneous solution. Subsequently, the solution was transferred into 50-ml Teflon-lined stainless steel autoclave and maintained at 200°C for 24 h to obtain the ZLFO NPs. The typical synthesis procedure can be shown by the following [20]:
Zn(NO3)2 · 6H2O + 0.02 La(OOCCH3)3 · 1.5H2O + 1.98 Fe(NO3)3 · 6H2O + C2H3NaO2 · 3H2O + C2H6O2 → ZnLa0.02Fe1.98(OOCH2CH3)8 · nH2O + NaNO3.
ZnLa0.02Fe1.98(OOCH2CH3)8 · nH2O will decompose above 200°C and produce ZLFO.
After the autoclave was cooled down to room temperature naturally, the precipitate obtained was separated by centrifugation, washed with distilled water and anhydrous ethanol several times, and subsequently dried at 80°C. The obtained ZLFO NPs were divided into two parts. One is diluted in the Al2O3 matrix and the other is undiluted.
ZLFO NPs were added to the solution of Al(NO3)3 · 9H2O and ethanol under sonicating with mass ratio of ZLFO:Al2O3, being 3:2. Then, the mixture was dried at 80°C. The undiluted and diluted ZLFO were both divided equally into four parts for annealing at 700°C, 800°C, 900°C, and 1,000°C for 2 h to obtain the final samples, which are hereafter referred to as UD700, UD800, UD900, and UD1000 for undiluted samples and D700, D800, D900, and D1000 for diluted samples, respectively.
The crystal structure was characterized by X-ray diffraction analysis using an X-ray diffractometer (XRD; DX-2000 SSC) with Cu Kα irradiation (λ = 1.5418 Å) from 10° to 80° with a step of 0.02°. The magnetic measurements were carried out by Quantum Design superconducting quantum interference device (SQUID) MPMS system(PPMS EC-II) (Quantum Design, San Diego, CA, USA). High-resolution transmission electron microscopy (HRTEM) (JEOL JEM-2100, JEOL, Akishima-shi, Tokyo, Japan) was used to observe the morphology, selected area electronic diffraction (SAED), and lattice fringes.
Results and discussion
Structural characterization
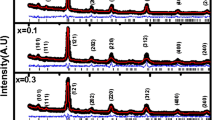
Firstly, the crystal structures of the obtained samples are analyzed. Figure 1 representatively shows XRD patterns for the as-prepared sample at 200°C (d), UD700 (c), UD1000 (b), and D1000 (a). According to the standard PDF card (No.79-1150), the diffraction peaks of the as-prepared sample can be indexed to the cubic spinel Zn ferrite with space group Fd3m (227) as reported in literature [21]. For the undiluted samples UD700 and UD1000, the intensity of diffraction peak increases with increasing the annealing temperature. The cell lattice parameter a of ZLFO can be obtained from , where a is the lattice parameter, θ the diffraction angle, λ the wavelength of the Cu Kα irradiation, and (HKL) the crystal plane index. The obtained lattice parameter a is 0.8339, 0.8424, and 0.8437 nm for the as-prepared, UD700 and UD1000, respectively. Correspondingly, the X-ray density d x can be calculated by the formula d x = 8 M/Na3, where M, N, and a are the molecular weight, Avogadro’s number, and lattice parameter, respectively [21]. The calculated d x value is 5.56, 5.39, and 5.37 g/cm3, comparable to 5.30 g/cm3 [22] and 5.35 g/cm3 for ZnFe2O4 [23]. The average crystallite grain size, calculated from the (311) XRD peak using Debye Scherrer’s formula, is 9.5, 10.7, and 38.8 nm.
For the diluted sample D1000, as shown in Figure 1a, the diffraction peaks of ZLFO cannot be observed, indicating that ZLFO was deeply embedded in the Al2O3 matrix. Several diffraction peaks of (012), (104), and (113) facets can be attributed to the reflection of Al2O3.
Figure 2 shows the TEM (a and d), SAED (b and e), and HRTEM (c and f) images of the samples UD700 (left panel) and D700 (right panel). The sample UD700 consists of particles with the size about 50 nm and some particles agglomerate to larger particles, as shown in Figure 2a. The SAED image in Figure 2b exhibits the distinct diffraction circles indicating the characteristic of polycrystalline. As seen in Figure 2c, the interfringe distances of 0.24, 0.31, and 0.47 nm correspond to the (222), (220), and (111) crystalline planes of ZLFO. The particles in the diluted sample D700 are conglomerated, as shown in Figure 2d, and the SAED image in Figure 2e appears some random diffraction dots from Al2O3. The HRTEM image exhibits distinct fringes with distances being 0.24 and 0.47 nm, also corresponding to (222) and (111) crystalline planes of ZLFO.
Magnetic properties
Figure 3 shows the magnetization (M) dependence on the applied magnetic field (H) (-2 T < H < +2 T), viz M(H) loops of all undiluted samples, measured at room temperature. The loop shape of the samples UD700, UD800, and UD900 resembles to that observed in ZnFe2O4 [24], being characteristic of the superparamagnetism (SPM) with very small coercivity H c (<80 Oe). The H c value is 62, 68, 80, and 52 Oe for UD700, UD800, UD900, and UD1000, respectively. As shown in the inset of Figure 3a, a distinct loop shift along H axis, i.e., the exchange-bias effect, can be observed, implying the existence of the surface spin layer (SSL) of the NP [25–28]. The M(H) loop of the sample UD1000 is almost a straight line, behaving as the paramagnetism (PM). The maximum magnetization (Mmax) at H = 2 T is 0.19, 0.18, 0.17, and 0.09 μ B /f.u. for UD700, UD800, UD900, and UD1000, respectively. The sample UD700 has the highest moment of 0.19 μ B /f.u., larger than the theoretical value of 0.1 μ B /f.u. for the 2% La3+ substituted bulk ZLFO. Such a larger Mmax value can also be assigned to the contribution of SSL [29]. With increasing the annealing temperature, the Mmax monotonously decreases. Therefore, the total moment of NPs are contributed by the moments of particle core (Mcore) and SSL (MSSL), i.e., the core-shell magnetization model.
The dependence of magnetization on magnetic field. The magnetization (M) dependence on the applied magnetic field (H) (-2 T < H < +2 T), vizM(H) loops of all undiluted samples UD700 (a), UD800 (b), UD900 (c), and UD1000 (d) measured at room temperature. The inset of (a) shows the magnified plot of loop in the low H region.
Figure 4 shows the magnetization measured in zero field-cooled (MZFC) and field-cooled (MFC) modes from 20 to 380 K in a field of 200 Oe for the samples UD700 (a) and UD1000 (b). For the sample UD700, a peak at 66 K in the MZFC curve is found, usually signifying the blocking temperature (T B ) [5, 30]. T B is associated with the particle size (V) and the effective anisotropy (Keff) through KeffV = 25k B T B , where k B is the Boltzmann constant. At the temperature above T B , the magnetic anisotropy energy barrier is overcome by the thermal energy, and the magnetic moment of each NP randomly fluctuates from one easy direction to another. Thus, the coercive field becomes small, and the small H c value may result from the surface anisotropy induced by the SSL [31]. This phenomenon is known as SPM. For the sample UD1000, T B locates at 76 K. The variation of T B can be assigned to the synergistic effects of the particle volume V and the effective anisotropy constant Keff. According to the XRD results, the crystallite size of UD1000 is 38.8 nm, larger than that (10.7 nm) of UD700, which may result in the increase of T B . According to the above discussion, the magnetic behavior at room temperature can be understood by a core-shell magnetization model, as schematically plotted in Figure 5.
As discussed above, the total moment (Mtotal) of a particle can be expressed as Mtotal = Mcore + MSSL. At 300 K, the ZLFO core is paramagnetic (PM) (with theoretical molecular moment of 0.1 μ B /f.u.). The sample annealed at low temperature (such as 700°C), as shown in Figure 5a, has the small core and the thick SSL. For the sample annealed at higher temperatures such as at 800°C and 900°C, as shown in Figure 5b, the core becomes larger and simultaneously, the SSL becomes thinner. While the sample is annealed at 1,000°C, the SSL becomes thinner or disappears, as shown in Figure 5c, and the M(H) loop behaves as PM with a linear loop shape in the field range used. Therefore, the gradual decrease in Mmax for the samples UD700, UD800, UD900, and UD1000 can be assigned to the decrease of MSSL [32].
Figure 6 shows the M(H) loops of all diluted samples with the magnetization value being normalized according to the mass ratio of ZLFO/Al2O3. The H c value is 13, 6, 191, and 391 Oe, and the Mmax value at H = 2 T is 0.23, 0.15, 0.05, and 0.04 μ B /f.u. The Mmax value also decreases with increasing annealing temperature, which can be assigned to the reduction of SSL thickness, as discussed in Figure 5. For the samples D700 and D800, their H c values are very small and the magnetization tends to saturate with increasing the H value, indicating the characteristic of the SPM. For the samples D900 and D1000, M(H) loops exhibit the distinct hysteresis and their H c values are much higher than those of correspondingly undiluted samples, which is characteristic of weak ferromagnetism.
Figure 7 shows the mass-normalized magnetization measured in zero field-cooled (MZFC) and field-cooled (MFC) modes from 20 to 380 K in a field of 200 Oe for the samples D700 (a) and D1000 (b). A peak in the MZFC curve appears at 150 K for the sample D700, and the MZFC and MFC curves almost overlap above 150 K. However, for the sample D1000, the MZFC and MFC curves do not overlap until about room temperature, much higher than 150 K, indicating the enhanced irreversibility. After annealed at 1,000°C, the interface between ZLFO and Al2O3 may form M-O-Al (M = Zn, La, and Fe in ZLFO) bonds. These bonds play a pinning role in the moment reverse, leading to the enhanced irreversibility and consequently resulting in the increase of coercivity, as observed in diluted Co and γ-Fe2O3 NPs [26, 33–36].
Conclusions
The ZLFO NPs were synthesized by the hydrothermal method. Then, some of ZLFO NPs were diluted in the Al2O3 matrix through the sol–gel method and the others were undiluted. The undiluted and diluted ZLFO were finally annealed at temperatures of 700°C, 800°C, 900°C, and 1,000°C to investigate the effects of surface spin and interface effects between ZLFO and Al2O3 on the magnetic parameters and magnetic ordering states.
For the undiluted samples, with increasing the annealing temperature, the thickness of the SSL decreases and ZLFO experiences SPM and PM according to the results of hysteresis loops. The maximum magnetization, Mmax, at 2 T of ZLFO decreases with increasing the annealing temperature which can be assigned to the decrease of SSL. For the diluted samples, the surface spin and the interface effect between ZLFO NPs and the Al2O3 matrix are the dominant factors affecting the magnetic properties. Our results indicate that in the nano-sized magnets with weak magnetism, the surface spin plays a crucial rule in the magnetic properties.
References
Liang YC, Hsia HY: Growth and crystallographic feature-dependent characterization of spinel zinc ferrite thin films by RF sputtering. Nanoscale Res Lett 2013, 8: 537(pp8).
Dong CH, Wang GX, Guo DW, Jiang CJ, Xue DS: Growth, structure, morphology, and magnetic properties of Ni ferrite films. Nanoscale Res Lett 2013, 8: 196(pp5).
Batoo KM, Ansari MS: Low temperature-fired Ni-Cu-Zn ferrite nanoparticles through auto-combustion method for multilayer chip inductor applications. Nanoscale Research Letters 2012, 7: 112(pp14).
Batoo KM: Microstructural and Mössbauer properties of low temperature synthesized Ni-Cd-Al ferrite nanoparticles. Nanoscale Res Lett 2011, 6: 499(pp7).
Yoon H, Lee JS, Min JH, Wu JH, Kim Y: Synthesis, microstructure, and magnetic properties of monosized MnxZnyFe3-x-yO4 ferrite nanocrystals. Nanoscale Res Lett 2013, 8: 530(pp5).
Zan FL, Ma YQ, Ma Q, Zheng GH, Dai ZX, Wu MZ, Li G, Sun ZQ, Chen XS: One-step hydrothermal synthesis and characterization of high magnetization CoFe2O4/Co0.7Fe0.3nanocomposite permanent magnets. J Alloys Compd 2013, 553: 79–85.
Xu YF, Ma YQ, Zhang X, Zheng GH, Dai ZX, Ma Q, Zan FL, Li G, Wu MZ: Effects of the Fe3+/Sr2+ molar ratio on the exchange-coupling of the composite and single-phase SrFe12O19. Eur Phy J App Phy 2013, 62: 10201(pp8).
Dormann JL, Fiorani D, Tronc E: Magnetic relaxation in fine-particle systems. Adv. Chem Phys 1997, 98: 283–294.
Zheng XG, Xu CN, Nishikubo K, Nishiyama K, Higemoto W, Moon WJ, Tanaka E, Otabe ES: Finite-size effect on Néel temperature in antiferromagnetic nanoparticles. Phys Rev B 2005, 72: 014464(pp8).
Tiwari SD, Rajeev KP: Signatures of spin-glass freezing in NiO nanoparticles. Phys Rev B 2005, 72: 104433(pp9).
Salabas EL, Rumplecker A, Kleitz F, Radu F, Schüth F: Exchange anisotropy in nanocasted Co3O4 nanowires. Nano Lett 2006, 6: 2977–2981. 10.1021/nl060528n
Winkler E, Zysler RD, Vasquez Mansilla M, Fiorani D: Surface anisotropy effects in NiO nanoparticles. Phys Rev B 2005, 72: 132409(pp4).
Yi JB, Ding J, Feng YP, Peng GW, Chow GM, Kawazoe Y, Liu BH, Yin JH, Thongmee S: Size-dependent magnetism and spin-glass behavior of amorphous NiO bulk, clusters, and nanocrystals: Experiments and first-principles calculations. Phys Rev B 2007, 76: 224402(pp5).
Tomou A, Gournis D, Panagiotopoulos I, Huang Y, Hadjipanayis GC, Kooi BJ: Weak ferromagnetism and exchange biasing in cobalt oxide nanoparticle systems. J Appl Phys 2006, 99: 123915. 10.1063/1.2207809
Punnoose A, Seehra MS: Hysteresis anomalies and exchange bias in 6.6 nm CuO nanoparticles. J Appl Phys 2002, 91: 7766. 10.1063/1.1447182
Zan FL, Ma YQ, Ma Q, Xu YF, Dai ZX, Zheng GH, Wu MZ, Li G: Magnetic and impedance properties of nanocomposite CoFe2O4/Co0.7Fe0.3 and single phase CoFe2O4 via one-step hydrothermal. J Amer Ceram Soc 2013, 96: 3100–3107.
Petracic O, Chen X, Bedanta S, Kleemann W, Sahoo S, Cardoso S, Freitas PP: Collective states of interacting ferromagnetic nanoparticles. J Magn Magn Mater 2006, 300: 192–197. 10.1016/j.jmmm.2005.10.061
Obaidat IM, Mohite V, Issa B, Tit N, Haik Y: Predicting a major role of surface spins in the magnetic properties of ferrite nanoparticles. Cryst Res Technol 2009, 44: 489–494. 10.1002/crat.200900022
Liang YC, Zhong H, Liao WK: Nanoscale crystal imperfection-induced characterization changes of manganite nanolayers with various crystallographic textures. Nanoscale Res Lett 2013, 8: 345–352. 10.1186/1556-276X-8-345
Iqbal MJ, Ashiq MN, Hernández-Gómez P, Muñoz JMM, Cabrera CT: Influence of annealing temperature and doping rate on the magnetic properties of Zr–Mn substituted Sr-hexaferrite nanoparticles. J Alloys Compd 2010, 500: 113–116. 10.1016/j.jallcom.2010.03.228
Zhao BB, Nan ZD: One-pot synthesis of ZnLa x Fe2-xO4 clusters without any template and their possible application in water treatment. J Mater Chem 2012, 22: 6581–6586. 10.1039/c1jm13406j
Martins P, Costa CM, Botelho G, Lanceros-Mendez S, Barandiaran JM, Gutierrez J: Dielectric and magnetic properties of ferrite /poly (vinylidene fluoride) nanocomposites. Mater Chem Phys 2012, 131: 698–705. 10.1016/j.matchemphys.2011.10.037
Slatineanu T, Iordan AR, Palamaru MN, Caltun OF, Gafton V, Leontie L: Synthesis and characterization of nanocrystalline Zn ferrites substituted with Ni. Mater Res Bull 2011, 46: 1455–1460. 10.1016/j.materresbull.2011.05.002
Xie TP, Xu LJ, Liu CL, Wang Y: Magnetic composite ZnFe2O4/SrFe12O19: Preparation, characterization, and photocatalytic activity under visible light. Appl Surf Sci 2013, 273: 684–691.
Peddis D, Cannas C, Piccaluga G, Agostinelli E, Fiorani D: Spin-glass-like freezing and enhanced magnetization in ultra-small CoFe2O4nanoparticles. Nanotech 2010, 21: 125705. 10.1088/0957-4484/21/12/125705
Kodama RH, Berkowitz AE: Mc Niff Jr EJ, Foner S: Surface spin disorder in NiFe2O4 nanoparticles. Phys Rev Lett 1996, 77: 394–397. 10.1103/PhysRevLett.77.394
Yoshii K: Magnetization reversal in TmCrO3. Mater Res Bull 2012, 47: 3243–3248. 10.1016/j.materresbull.2012.08.005
Ma Q, Ma YQ, Zan FL, Xu YF, Zheng GH, Dai ZX, Wu MZ, Li G: Complex exchange anisotropy behavior in Co3O4-Ni0.6Zn0.4Fe2O4 composite with different Co3O4 content. Mater Res Bull 2014, 51: 381–388.
Benitez MJ, Petracic O, Salabas EL, Radu F, Tüysüz H, Schüth F, Zabel H: Evidence for core-shell magnetic behavior in antiferromagnetic Co3O4 nanowires. Phys Rev Lett 2008, 101: 097206.
Ceylan A, Hasanain SK: Ismat Shah S: Experimental observations of field-dependent activation of core and surface spins in Ni-ferrite nanoparticles. J Phys Condens Matter 2008, 20: 195208. 10.1088/0953-8984/20/19/195208
Veronica BG, Regino SP, María J, Veronica BG, Regino SP, María J: Superparamagnetism and interparticle interactions in ZnFe2O4 nanocrystals. J Mater Chem 2012, 22: 2992–3003. 10.1039/c1jm14856g
Sabsabi Z, Vernay F, Iglesias O, Kachkachi H: Interplay between surface anisotropy and dipolar interactions in an assembly of nanomagnets. Phys Rev B 2013, 88: 104424(pp12).
Rong CB, Zhang HW, Chen RJ, He SL, Shen BG: The role of dipolar interaction in nanocomposite permanent magnets. J Magn Magn Mater 2006, 302: 126–136. 10.1016/j.jmmm.2005.08.026
Chen JP, Sorensen CM, Klabunde KJ, Hadjipanayis GC: Enhanced magnetization of nanoscale colloidal cobalt particles. Phys Rev B 1995, 51: 11527–-11532.
Respaud M, Broto JM, Rakoto H, Fert AR, Thomas L, Barbara B, Verelst M, Lecante E, Snoeck P, Mosset A, Osuna J, Ely TO, Amiens C, Chaudret B: Surface effects on the magnetic properties of ultrafine cobalt particles. Phys Rev B 1998, 57: 2925–2935. 10.1103/PhysRevB.57.2925
Tronc E, Ezzir A, Cherkaoui R, Chanéac C, Noguès M, Kachkachi H, Fiorani D, Testa AM, Grenèche JM, Jolivet JP: Surface-related properties of γ-Fe2O3 nanoparticles. J Magn Magn Mater 2000, 221: 63–79. 10.1016/S0304-8853(00)00369-3
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos. 11174004, 51471001, and 11204001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
STX synthesized ZLFO NPs, performed the measurements, analyzed the magnetic properties, and wrote the manuscript. YQM gave the instruction to this research work, analyzed the results, and wrote the manuscript. YFX, XS, and BQG measured and analyzed the magnetic properties of nanoparticles. GHZ and ZXD supervised the overall study. All authors contributed to discussing the results and writing manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xu, S., Ma, Y., Xu, Y. et al. The effects of surface spin on magnetic properties of weak magnetic ZnLa0.02Fe1.98O4 nanoparticles. Nanoscale Res Lett 9, 545 (2014). https://doi.org/10.1186/1556-276X-9-545
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1556-276X-9-545