Abstract
A hybrid nanolaminates consisting of Al2O3/ZrO2/alucone (aluminum alkoxides with carbon-containing backbones) grown by atomic layer deposition (ALD) were reported for an encapsulation of organic light-emitting diodes (OLEDs). The electrical Ca test in this study was designed to measure the water vapor transmission rate (WVTR) of nanolaminates. We found that moisture barrier performance was improved with the increasing of the number of dyads (Al2O3/ZrO2/alucone) and the WVTR reached 8.5 × 10−5 g/m2/day at 25°C, relative humidity (RH) 85%. The half lifetime of a green OLED with the initial luminance of 1,500 cd/m2 reached 350 h using three pairs of the Al2O3 (15 nm)/ZrO2 (15 nm)/alucone (80 nm) as encapsulation layers.
Similar content being viewed by others
Background
Active matrix organic light-emitting diodes (AM OLED) was focused as the next-generation display since its great advantages, vivid full color ,high brightness, low power consumption, fast response time, and suitable for flexible display [1]. In terms of OLEDs technology, the encapsulation process is a core technology influencing both the lifetime and reliability of OLEDs. The devices need encapsulation materials to protect it from water and oxygen. Thin film encapsulation (TFE) is considered as one of the most potent methods to ensure for protection from moisture and oxygen penetration in electronic devices [2,3]. Metal oxide thin film has been the choices for TFE materials such as aluminum oxide (Al2O3) and zirconium oxide (ZrO2) and titanium oxide (TiO2) grown by atomic layer deposition (ALD) since their superior protection forms moisture [4-6]. Recently, several researches have focused on the multilayered nanolaminate structure which comprised of alternating layer of different materials that have individual layer of nanometer-scale thickness [7,8].
However, inorganic materials have critical weaknesses such as cracking and pinhole defects in the layer surface [9,10]. To solve these problems, alternating inorganic and organic layer pairs is suggested as an encapsulation solution for OLED devices. Generally, in the multilayer structure, the role of the organic layer is known to decouple defects in the oxide layers, thereby preventing propagation of defects through the multilayer structure. ALD and molecular layer deposition (MLD) processes can deposit smooth, conformal, and pinhole-free films. Recently, an ALD/MLD combination structure has been proposed because ALD/MLD multilayers have very good film integrity which causes them to have advanced performance for thin film encapsulation [11-13].
In this study, we report on thin encapsulation layers deposited by ALD at 85°C. For the thin encapsulation layers, various nanolaminate structures consisting of Al2O3/ZrO2/alucone (aluminum alkoxides with carbon-containing backbones) were tested to determine the best structure producing the long lifetime devices based on the fact that nanolaminate structures significantly enhanced the lifetime by suppressing the formation of both microscopic voids and nanocrystals that could exist in an Al2O3 or ZrO2 single layer.
Methods
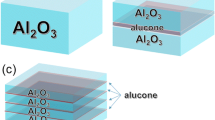
The encapsulation structure of OLEDs is shown in Figure 1, and OLED devices were fabricated by conventional vacuum deposition system. Encapsulation layers were deposited by ALD system. An indium tin oxide (ITO, 10 ohm, 150 nm)-coated glass substrates were used and cleaned with a detergent solution, deionized water, and acetone. After treated for 10 min by plasma, the substrate was transferred to vacuum deposition system. The OLEDs were fabricated by sequentially depositing the following organic layer, which were 4,4′,4″-tris-N-naphthyl-N-phenylamino-triphenylamine (40 nm) as a hole injection layer, N,N′-bis-(naphthyl)-N,N′-diphenyl-1,1′-biphenyl-4,4′-diamine (20 nm) as a hole transport layer, tris(8-hydroxyquinolinato)aluminum (Alq3) doped (20 nm) as a light-emitting layer, and Alq3(30 nm) as an electron transport layer using a shadow mask. In addition, lithium fluoride (LiF, 0.5 nm) and aluminum (Al, 100 nm) as electron injection layer and cathode, respectively, were evaporated onto the organic layer using a metal shadow mask. The typical deposition rates were 0.5 Å/s, 0.1 Å/s, and 5.0 Å/s for organic materials, LiF, and Al, respectively.
ALD Al2O3 films were fabricated using H2O and trimethylaluminum (TMA) as precursors at 85°C. ALD ZrO2 films were fabricated using H2O and tetrakis (dimethylamido) zirconium (TDMAZ) as precursors at 85°C. Nitrogen (N2, 99.999%) was used as a carrier gas on the TMA/TDMAZ and as the purge gas. Alucone films were grown using TMA and ethylene glycol (EG) at 85°C. The process pressure of ALD and MLD was 500 mTorr. In addition, Al2O3 films were deposited using 100 ms of TMA pulse, 7 s of N2 purge time, 100 ms of H2O pulse, and 7 s of purge, while ZrO2 films were deposited using 250 ms of TDMAZ pulse, 10 s of N2 purge, 200 ms H2O pulse, and 10 s of purge time, and alucone films were deposited using 200 ms of TMA pulse, 15 s of N2 purge, 300 ms of EG flow with carrier gas, and 20 s of N2 purge.
The general reactions between the metal alkyl and the diol of ALD Al2O3 film and MLD alucone film can be written as follows (Figure 2). The thicknesses of the ALD film were measured by spectroscopy ellipsometry at wavelengths from 245.57 to 1,664.00 nm.
Results and discussion
Surface morphology
We have investigated the surface morphology of the encapsulation film using atomic force microscopy (AFM) measurement with a trapping mode on the silicon wafer substrate. Figure 3a,b,c shows the surface topography of the single Al2O3, Al2O3/ZrO2, and Al2O3/ZrO2/alucone, respectively. The root-mean-square (RMS) surface roughness of the single Al2O3, Al2O3/ZrO2, and Al2O3/ZrO2/alucone layers was 1.12, 1.31, and 0.83 nm separately. The lower roughness of Al2O3/ZrO2/alucone film indicates that the introduction of alucone can make the surface smoother. Moreover, we have observed that the surface topography of the Al2O3 film was similar to cloth-like while that of the ZrO2 was grain-like, revealing that the Al2O3 layer is more suitable to deposit directly onto the cathode before the ZrO2 layer.
Optical transmission
The used Al2O3, ZrO2, and alucone films are highly transparent. We have measured the light transmission characteristics of the encapsulation film, as shown in Figure 4. The light transmission of the Al2O3 and ZrO2 layer is above 95%. In addition, it is worth to note that the light transmission of both Al2O3/ZrO2/alucone (one pair) and Al2O3/ZrO2/alucone (three pairs) are higher than those of the Al2O3 and ZrO2 layers at the range of 400 to 700 nm. The addition of alucone can effectively prevent the light scattering in visible wavelength (450 to 650 nm) due to the its lower surface RMS. Although the transmission below 450 nm is indeed lower than Al2O3 and Al2O3/ZrO2 films, the transmission increases due to the decreasing light scattering (Additional file 1: Figure S1). This demonstrates that organic/inorganic film may be suitable for the visible electroluminescent emission of the top-emitting OLEDs.
WVTR result
The Ca test in this study was designed to the water vapor transmission rate (WVTR) of nanolaminates. The amount of Ca oxidation was used to calculate the amount of water vapor using the resistivity of Ca films. We utilized the 200-nm-thick Ca layer, which is close to the normal thickness of an aluminum cathode in OLED. To prevent the Ca film from contacting with water and oxygen, the ALD system and the equipment for Ca fabrication were connected with a glove box filled with nitrogen gas. WVTR of barriers was calculated as the following equation [14-17]:
where n is the molar equivalent of the degradation reaction, M (reagent) and M (Ca) are the molar masses of the permeating reagent and Ca, ρis the Ca resistivity, and δ is the density of Ca. Figure 5 shows the barrier performance of different thin films. The WVTR values of ALD-grown moisture barrier films with three pairs of Al2O3/ZrO2/alucone are as low as 8.5 × 10−5 g/m2/day at 25°C, 85% relative humidity (RH).
Lifetime of OLED
We have measured the lifetime of OLEDs encapsulated by various ALD thin film structure. For devices A and B, the passivation film were Al2O3 (30 nm) and Al2O3 (15 nm)/ZrO2 (15 nm), while devices C and D used Al2O3 (15 nm)/ZrO2 (15 nm)/alucone (80 nm) and three pairs of Al2O3 (15 nm)/ZrO2 (15 nm)/alucone (80 nm). We compared the evolution of the luminance devices A, B, C, and D as shown in Figure 6. It was shown that the lifetime of device A with single Al2O3 layer decreased obviously than device B with Al2O3 (15 nm)/ZrO2 (15 nm). The Al2O3 layer acted as a better moisture barrier than ZrO2 at the same thickness. The Al2O3 layer functioned as a better moisture barrier than ZrO2 at the same thickness. The WVTR of Al2O3 and ZrO2 were 2.38 × 10−3 g/m2/day and 4.5 × 10−3 g/m2/day when fixing the film thickness by 30 nm. A denser ZrAlxOy-aluminate phase with higher packing density could be formed at the interfaces between Al2O3 and ZrO2, leading to a densification at the Al2O3/ZrO2 interfaces [18,19]. Because the permeation rate for gasses such as water vapor depends on the density of the material, even a small amount of water can enter into the device through the thin film.
Device D with three pairs of inorganic/organic hybrid layers can drastically improve the lifetime of OLED because the organic layer (alucone) may increase the water vapor diffusion path in the film and decrease the diffusion speed (or diffusivity) by trapping water vapor chemically. Generally, it is called a ‘tortuous path’, which is possibly governed by the strong dependence of the WVTR on the organic layer thickness [10,20,21]. The half lifetime of a green OLED with the initial luminance of 1,500 cd/m2 reached 380 h using three pairs of the Al2O3 (15 nm)/ZrO2 (15 nm)/alucone (80 nm) as encapsulation layers.
Conclusions
Hybrid Al2O3/ZrO2/alucone thin film encapsulation structure can obviously improve barrier performance. The WVTR is as low as 8.5 × 10−5 g/m2/day at 25°C, 85% RH. A half lifetime of 380 h at initial luminance of 1,500 cd/m2 for a green organic light-emitting diode with developed TFE technology has been achieved.
Abbreviations
- ALD:
-
Atomic layer deposition
- OLED:
-
Organic lighting emitting diodes
- WVTR:
-
Water vapor transmission rate
- RMS:
-
Root-mean-square
References
Lee YG, Choi Y-H, KeeaLee IS. Thin-film encapsulation of top-emission organic light-emitting devices with polyurea/Al2O3 hybrid multi-layers. Organic Electron. 2009;10(7):1352–5.
Ghosh AP, Gerenser ALJ, Jarman CM, Fornalik JE. Thin-film encapsulation of organic light-emitting devices. Appl Phys Lett. 2005;86(22):223503.
Liu K-C, Cheng H-L, Tsai J-R, Chiang Y-L, Hsieh Y-C, Jan D-J. Investigation of SiOxCy film as the encapsulation layer for full transparent OLED using hollow cathode discharge plasma at room temperature. Thin Solid Films. 2010;518(22):6195–8.
Meyer J, Gorrn P, Bertram F, Hamwi S, Winkler T, Johannes H-H, et al. Al2O3/ZrO2 nanolaminates as ultrahigh gas-diffusion barriers - a strategy for reliable encapsulation of organic electronics. Adv Mater. 2009;21(18):1845–9.
Park J, Noh Y-Y, Huh JW, Lee J, Chu H. Optical and barrier properties of thin-film encapsulations for transparent OLEDs. Organic Electron. 2012;13(10):1956–61.
Zhao C, Richard O, Bender H, Caymax M, De Gendt S, Heyns M. Miscibility of amorphous ZrO[sub 2]–Al[sub 2]O[sub 3] binary alloy. Appl Phys Lett. 2002;80(13):2374.
Stoldt CR, Bright VM. Ultra-thin film encapsulation processes for micro-electro-mechanical devices and systems. J Phys D Appl Phys. 2006;39(9):R163–70.
Liao Y, Yu F, Long L, Wei B, Lu L, Zhang J. Low-cost and reliable thin film encapsulation for organic light emitting diodes using magnesium fluoride and zinc sulfide. Thin Solid Films. 2011;519(7):2344–8.
Fumagalli L, Binda M, Lopez IS, Natali D, Sampietro M, Ferrari S, et al. Multi layer structure for encapsulation of organic transistors. Organic Electron. 2009;10(4):692–5.
Graff GL, Williford RE, Burrows PE. Mechanisms of vapor permeation through multilayer barrier films: lag time versus equilibrium permeation. J Appl Phys. 2004;96(4):1840.
Puurunen RL. Surface chemistry of atomic layer deposition: a case study for the trimethylaluminum/water process. J Appl Phys. 2005;97(12):121301.
Hana YC, Kima E, Kima W, Imb H-G, Bae B-S, Choi KC. A flexible moisture barrier comprised of a SiO2-embedded organic–inorganic hybrid nanocomposite and Al2O3 for thin-film encapsulation of OLEDs. Organic Electron. 2013;14(6):1435–40.
Katsumi T, Ishimori H, Onikata M, Fukagawa R. Long-term barrier performance of modified bentonite materials against sodium and calcium permeant solutions. Geotextiles Geomembranes. 2008;26(1):14–30.
Carcia PF, McLean RS, Reilly MH, Groner MD, George SM. Ca test of Al[sub 2]O[sub 3] gas diffusion barriers grown by atomic layer deposition on polymers. Appl Phys Lett. 2006;89(3):031915.
Carcia PF, McLean RS, Reilly MH. Permeation measurements and modeling of highly defective Al[sub 2]O[sub 3] thin films grown by atomic layer deposition on polymers. Appl Phys Lett. 2010;97(22):221901.
Park J-S, Chae H, Chung HK, Lee SI. Thin film encapsulation for flexible AM-OLED: a review. Semiconductor Sci Technol. 2011;26(3):034001.
Schubert S, Klumbies H, Müller-Meskamp L, Leo K. Electrical calcium test for moisture barrier evaluation for organic devices. Rev Sci Instrum. 2011;82(9):094101.
Seo S-W, Jung E, Chae H, ChoaSeo SM. Optimization of Al2O3/ZrO2 nanolaminate structure for thin-film encapsulation of OLEDs. Organic Electron. 2012;13(11):2436–41.
Meyer J, Schneidenbach D, Winkler T, Hamwi S, Weimann T. Reliable thin film encapsulation for organic light emitting diodes grown by low-temperature atomic layer deposition. Appl Phys Lett. 2009;94(23):233305.
Park J, Ham H, Park C. Heat transfer property of thin-film encapsulation for OLEDs. Organic Electron. 2011;12(2):227–33.
Seo S-W, Chae H, Sang Joon S, Ho Kyoon C, Sung MC. Extremely bendable thin-film encapsulation of organic light-emitting diodes. Appl Phys Lett. 2013;102(16):161908.
Acknowledgements
This work was funded by National Key Basic Research Program of China (2015CB655005) and Science and Technology Commission of Shanghai Municipality Program (14DZ228090).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
The work presented here was carried out in collaboration between all authors. All authors have contributed to, seen, and approved the manuscript.
Additional file
Additional file 1: Figure S1.
The light scattering in visible wavelength (450-650 nm) for different type of encapsulated film.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Zhang, H., Ding, H., Wei, M. et al. Thin film encapsulation for organic light-emitting diodes using inorganic/organic hybrid layers by atomic layer deposition. Nanoscale Res Lett 10, 169 (2015). https://doi.org/10.1186/s11671-015-0857-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11671-015-0857-8