Abstract
Wurtzite CuInS2 nanoleaves were synthesized by Cu1.94S-assisted growth. By observing the evolution of structures and phases during the growth process, Cu1.94S nanocrystals were found to be formed after uninterrupted oxidation and sulfidation of copper nanoparticles at the early stage, serving as catalysts to introduce the Cu and In species into CuInS2 nanoleaves growth for inherent property of fast ionic conductor. The obtained CuInS2 nanoleaves were characterized by scanning transmission electron microscopy, transmission electron microscopy, fast Fourier transform, X-ray diffraction, and energy dispersive X-ray spectroscopy mapping. The enhancement of photoresponsive current of CuInS2 nanoleaf film, evaluated by I-V curves of nanoleaf film, is believed to be attributed to the fast carrier transport benefit from the nature of single crystalline of CuInS2 nanoleaves.
Similar content being viewed by others
Background
Ternary I-III-VI2 groups of compounds are important players in solar energy-harvesting materials [1–3]. Among them, CuInS2 is a direct gap semiconductor with a bulk band gap of approximately 1.5 eV and a high extinction coefficient of around 105 cm−1 [4, 5]. It is noteworthy that bulk CuInS2 at room temperature has the chalcopyrite structure, whereas CuInS2 nanocrystals can be additionally synthesized in zincblende and wurtzite structure [6]. Since Pan et al. [7] reported the colloidal synthesis of CuInS2 nanocrystals with wurtzite structure via hot injection, numerous research works on the field of metastable wurtzite CuInS2 nanocrystals have been reported [4], including the synthesis, phase transformation, and photovoltaic application. Kolny-Olesiak et al. [8] demonstrated the phase transforming from Cu2S to wurtzite CuInS2 nanocrystals.
The wurtzite CuInS2 is constructed as randomly distributed copper and indium over the cation sites of the wurtzite ZnS lattice [6]. The cation disorder allows flexibility of the stoichiometry and a tunable Fermi energy over a wide range, which feature particularly in wurtzite CuInS2 nanocrystals for the following device fabrication [9]. While most reports describe the preparation of CuInS2 nanocrystals, limited work is available for one-dimensional CuInS2 nanomaterials [4, 10]. Semiconductor nanomaterials in one-dimensional morphology provide ideal models to study the relationship between electrical transport, optical, and other properties with dimensionality and size confinement [11–13]. Specifically, one-dimensional nanomaterials could offer continuous charge carrier transport pathways and efficiently promote charge separation, which makes them highly attractive for photocatalytic and photovoltaic applications [14–16]. Thus, one-dimensional nanomaterials comprise an important class of nanomaterials used in electronic and photoelectronic devices, including field-effect transistors, energy harvesting, and sensors [12, 17].
To synthesize one-dimensional nanomaterials in solution, several mechanisms have been developed [11], including catalyst-assisted growth, template-directed growth, and oriented attachment growth. Among them, catalyst-assisted growth [18] exhibited wonderful features to acquire one-dimensional nanomaterials with high crystallinity, tolerating big lattice mismatch between catalysts and targeted nanomaterials. During the growth process, catalyst either formed a liquid eutectic in solution-liquid-solid growth [19], which induces nanowire formation after supersaturation, or enables solid-phase diffusion in supercritical-fluid-liquid-solid growth in which the catalysts remain solid [20]. In these researches, metallic bismuth and indium nanocrystals usually acted as the catalysts [21]. Recently, sulfide Ag2Se and Cu2S nanocrystals have also been found to be the effective catalysts in the synthesis of one-dimensional nanomaterials for the intrinsic nature of fast ionic conductor [22, 23]. Wang et al. [22] reported Ag2Se nanocrystals could be used as catalysts for the growth of semiconductor heterostructures, such as dimeric Ag2Se-CdSe and trimeric Ag2Se-CdSe-ZnSe. Further, Tang et al. [24] successfully fabricated Cu2S-In2S3 heterostructures by djurleite Cu1.94S-assisted growth model, in which the catalyst underwent transformations in crystal structure and composition. Accordingly, Wang et al. [25] proposed the novel solution-solid-solid mechanism for nanowire growth catalyzed by superionic (fast ionic) conductor nanocrystals. By using solution-solid-solid growth, Ag2S-CdS, Cu2S-ZnS, and Ag2Se-ZnSe heterostructures were prepared [26, 27]. In the growth process of one-dimensional nanomaterials, Ag2S and Cu2S nanocrystals were usually decomposed from single-source molecular precursors and used as catalysts. Then, the target species dissolved into the catalysts and dissolved out after supersaturation. The complicated process in these cases makes one aware that further investigation is needed, for the solubility and fluidity of intermediate species in the catalysts and the supersaturation and condensation of target substances are unique [27, 28]. Thus, there is much room in the exploration of catalysts for the growth of the desired nanomaterials.
Here, we report the catalyst-assisted growth of wurtzite CuInS2 nanoleaves in solution by using commercial copper nanoparticles as staring materials. The transformation from copper nanoparticle to copper oxide in oxygen atmosphere underwent quickly at elevated temperature, and then to copper sulfide Cu1.94S with the presence of dodecanethiol. Detailed investigation on the growth by monitoring the structures and morphologies of the nanoleaves during the process implied that the formed Cu1.94S nanocrystals played the catalytic roles for the CuInS2 nanoleaf growth. The structure and composition of CuInS2 nanoleaves were also investigated by transmission electron microscopy (TEM), X-ray diffraction (XRD), and energy-dispersive X-ray spectroscopy (EDS). Furthermore, the photoresponsive characteristics of the CuInS2 nanoleaf film were also evaluated.
Methods
Materials
All chemicals were used as received without further purification. Sodium diethyldithiocarbamate trihydrate (Na(dedc), 99 %), chloroform (99.9 %), and n-hexane (95 %) were obtained from J&K, indium nitrate (In(NO3)3, 99.9 %) from ABCR, oleylamine (OA, C18 content 80–90 %) from Acros, and copper nanoparticle (99.9 %) and 1-dodecanethiol (DT, 98 %) from Alfa.
Synthesis of In(dedc)3 Precursors
In a typical synthesis of In(dedc)3, 3 mmol Na(dedc) and 1 mmol In(NO3)3 were dissolved in 50 mL ionized water, respectively. Then, In(NO3)3 aqueous solution was mixed with Na(dedc) solution by drop adding, washed three times at least with ionized water and ethanol followed by drying. As-synthesized precursors were stored in desiccator at room temperature.
Synthesis of CuInS2 Nanoleaves
In a typical synthesis of CuInS2 nanoleaves, 0.1 mmol (6.4 mg) copper nanoparticles and 0.05 mmol (28.0 mg) In(dedc)3 were dispersed in 6.0 mmol (2.0 mL) OA and 16.5 mmol (4.0 mL) DT-loaded flask under magnetic stirring. Then, the flask was vacuumed and filled with oxygen. The procedures were repeated three times and the oxygen flow was maintained during the following reaction. The flask containing the mixture was immersed in an oil bath at 180 °C. The heated solution in the flask showed the color evolution within 1 min, from transparent yellow to light brownish red, implying the formation and decomposition of the Cu-DT complex. After keeping the mixture at the temperature for 60 min, the resulting solution was cooled to room temperature and the samples were washed with n-hexane followed by further centrifugation. Aliquots were taken out during the synthesis for monitoring the size and shape evolution of nanoleaves.
Characterization
The obtained crystalline phases were identified using powder XRD (Bruker, D8 advance, Cu Ka radiation using a curved graphite receiving monochromate), with a step of 0.02° at a speed of 4°/min. The simulated XRD patterns of CuInS2 were obtained by using CrystalMaker 2.5.5 programs. Morphology analyses were undertaken using scanning transmission electron microscopy (STEM, FEI Nova NanoSEM 200). TEM, high-angle annular dark-field (HAADF), STEM, and EDS were performed on JEOL 2100F microscope. The samples for TEM, HAADF-STEM, and STEM-EDS were collected by placing a drop of dilute solution of sample in hexane onto carbon-film-supported nickel grids. Composition analysis was performed by EDS (oxford INCA). The two parallel gold electrodes on silicon substrate with quartz layer were used to evaluate electrical property of CuInS2 nanoleaves. The interval and length of the two gold electrodes is 5 and 100 μm. Sample was made by drop-casting nanoleaves in chloroform onto the substrate. Annealing process was conducted at 400 °C to remove the attached ligands. The current-voltage characteristics were recorded using a Keithley 4200 Source Meter in the dark and under illumination. The scan voltage was tuned from −10 to 10 V.
Results and Discussion
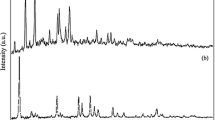
Typical STEM micrographs of the resultant CuInS2 nanomaterials were shown in Fig. 1. The representative micrographs of the as-synthesized samples indicate that the CuInS2 nanomaterials have a leaf shape, about 500 nm in length and 100 nm in width. The nanoparticle is observed at the tip of almost every nanoleaf (red circles in Fig. 1b), which is the typical morphology of catalyst-assisted growth. The crystal structure of CuInS2 nanoleaves is determined as hexagonal wurtzite phase, originated from wurtzite ZnS by randomly replacing zinc ions with copper or indium ions [7, 8]. The diffraction pattern of wurtzite CuInS2 is simulated (a = 3.9080 Å, c = 6.4250 Å) and listed as reference line in Fig. 1c, for the standard card has not been established. The lattice-resolved high-resolution TEM micrograph (Fig. 1d) shows the single crystal nature of the individual nanoleaves with lattice spacing of 3.38 Å. Accordingly, the (100) plane of the wurtzite CuInS2 is resolved. The regularity of lattice spacing in the nanoleaves is also seen from the line profile as inset in Fig. 1d, enclosed from the yellow rectangle area. The distance of 6.76 nm can be represented as 20 × 3.38 Å, which is the lattice spacing of (100) plane in CuInS2.
a, b STEM micrographs of CuInS2 nanoleaves. The red circles indicate the typical end terminal of the nanoleaves. c XRD pattern of as-synthesized CuInS2 nanoleaves. The reference line below is simulated according to wurtzite CuInS2 lattice constants (a = 3.9080 Å, c = 6.4250 Å). d HRTEM micrograph of CuInS2 nanoleaves. Inset represents line profile integrated along the region enclosed in the yellow rectangle area
Figure 2 displays the STEM micrograph and EDS spectrum of CuInS2 nanoleaves. The composition of the nanoleaves was mainly identified as Cu, In, and S elements. Chemical analyses with nanoscale spatial resolution are performed to clarify the sample compositions and elemental distributions in individual. The 2D-projected elemental maps for three atoms, shown in Fig. 2d–f and Additional file 1: Fig. S1, demonstrate the reasonable separated distribution of three atoms among the nanoleaves. Indicated by yellow circles in Fig. 2b, d–f and Additional file 1: Fig. S1, enhanced copper content in head part of nanoleaves is thought to be attributed to copper sulfide catalyst and will be discussed later. The composition of the head part in the nanoleaf was identified as Cu and S elements with ratio of 61/39. Meanwhile, element ratio 26/29/45 of Cu/In/S was identified in the body part of nanoleaf. Also, line scan across the single CuInS2 nanoleaf testified the difference between head and body parts (Additional file 1: Fig. S2 and S3). Copper content has considerable high ratio in the head part than body part, while indium content could be negligible in the head part compared with that in the body part.
HAADF-STEM micrographs of CuInS2 nanoleaves. a HAADF micrograph. b The composite STEM-EDS micrograph. c EDS spectrum collected from CuInS2 nanoleaves. Si element peak attributed to EDS detector and Cr, Fe, and Ni element peaks attributed to nonmagnetic nickel grids. d–f Cu, In, and S of STEM-EDS elemental maps of CuInS2 nanoleaves
A typical HRTEM micrograph of the tip in CuInS2 nanoleaf is shown in Fig. 3a. The interface between head and body parts in the nanoleaf, indicated by the parallel dotted lines, is clearly revealed. The two FFT patterns corresponding to head and body parts are noticeably dissimilar and are indexed to monoclinic Cu1.94S and wurtzite CuInS2, respectively. Lattice spacing with 3.40 Å in the head and 3.38 Å in the body is corresponded to the (042) plane of Cu1.94S crystal and (100) plane of CuInS2 crystal, respectively. An interplanar distance analysis based on the HRTEM and FFT pattern shown in Fig. 3 suggests that the (201̄0) plane of CuInS2 nanoleaf epitaxially attaches to the (046) plane of the Cu1.94S head, which demonstrates the grain boundary is composed of (21̄0) planes of CuInS2 and (046) planes of Cu1.94S. Based on above analysis, an atomic packing model that depicts the epitaxial attachment of the CuInS2 body to Cu1.94S head is proposed. The corresponding interface orientation relationships between the CuInS2 and Cu1.94S are (010)CuInS2||(042̄)Cu1.94S and [001]CuInS2|| [1̄00]Cu1.94S, respectively. The values of lattice mismatch are [(3.38–3.40)/3.40 × 100 %] = −0.59 % for (010)CuInS2||(042̄)Cu1.94S direction, and [(3.38–3.36)/3.38 × 100 %] = 0.59 % for (001)CuInS2||(8̄00)Cu1.94S direction, respectively. The relatively small lattice mismatch along both directions enables epitaxial growth of CuInS2 nanoleaf on Cu1.94S head.
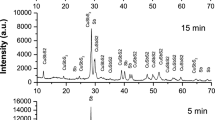
To explore the growth of CuInS2 nanoleaves, the phase and morphological evolution of samples at different reaction stages was investigated by XRD and TEM (Figs. 4 and 5). Metal copper and indium hydroxide were the main phase of the solid product within 15 s after the reaction system immersed into the oil bath at 180 °C, and the latter was originated from the reaction between oleylamine and indium precursors. The signal of djurleite Cu1.94S emerged as the reaction lasted for 60 s. The coexistence of wurtzite CuInS2, indium hydroxide, and djurleite Cu1.94S was observed in the product after 120 s. It is supposed that djurleite Cu1.94S catalyzed the growth for the nanoleaves. By contrast, copper phase kept unchanged at the early stage under nitrogen atmosphere (the absence of oxygen) in the controlled experiment. The transformation from copper nanoparticle to copper oxide in oxygen atmosphere underwent at elevating temperature process, and then to Cu1.94S rapidly after the reaction with dodecanethiol. It is thought that sulfidation has higher reaction rate than oxidation, though the latter possesses the priority, which also explains the fail trial on the confirmation of copper oxide by XRD analysis. In vapor system, the role of oxygen pressure during the transformation from metal copper to copper sulfide has been clarified [29, 30]. Monoclinic Cu2S nanowire arrays were grown on Cu foil substrates with the aid of H2S gas. In consideration that catalytic roles played by Cu1.94S were validated only if wurtzite CuInS2 and djurleite Cu1.94S coexist, the sample at 2 min was chose as the target. As shown in Fig. 5a, the samples at the early stage appear as acorns. Lattice-resolved HRTEM micrographs (Fig. 5b) clearly reveal the interface between the head and body parts of samples, indicated by the yellow dotted lines. With the growth proceeding (Fig. 5c–e), the body parts of CuInS2 samples grew with the gradual increase of length, whereas the Cu1.94S head parts and the interfaces kept.
The Cu1.94S head part in CuInS2 nanomaterial is deduced to be catalyst, which is the typical role in the synthesis of one-dimensional nanomaterial by the mechanisms of solution-liquid-solid and vapor-liquid-solid growth [19, 31]. Recently, superionic conductor nanocrystals, such as Ag2S, Ag2Se, and Cu2S, are found to be efficient catalysts in the growth of nanowires and heteronanostructures for their intrinsic nature [22, 25–27, 32]. Also, new mechanism has been proposed as solution-solid-solid mode [25]. The superionic conductor catalysts have enough cation vacancies in their lattice with high cation mobility in the rigid anionic sublattice. It has been demonstrated that djurleite Cu1.94S nanocrystal can catalyze the growth of Cu2S-In2S3, Cu1.94S-CdS, and Cu2S-PbS heterostructures for the intrinsic cationic deficiencies [23, 24, 33, 34]. In the present work, the catalyst Cu1.94S nanocrystal introduces Cu(I) and In(III) species into the vacant sites of the crystal lattice, then condenses and crystallizes successively after saturation from the favorable facet of the catalyst to minimize the interfacial energy. As calculated from the proposed atomic packing model, lattice mismatches are as small as 0.59 %. We can deduce that grain boundary with low interfacial energy between djurleite Cu1.94S head and wurtzite CuInS2 body is formed.
Comparatively, size enlargement of Cu1.94S, from 10 nm in width at 2 min (Fig. 5a, b) to 100 nm at 60 min (Fig. 3a), provided further evidence for catalyst-assisted growth of CuInS2 nanoleaves. If seed-mediated growth model is employed in the present system, the size of Cu1.94S should be stable as the targeted material only grows epitaxially on the specific facet of Cu1.94S seed. From this point, the growth of the targeted materials just involves the first atomic layer epitaxial growth on seed, then transforms into conventional crystal growth. Thus, the subsequent growth of CuInS2 nanoleaves by seed-mediated growth makes no difference to the seed, either in composition or in size.
To further evaluate the optoelectronic properties of the CuInS2 nanoleaves, thin films by drop-casting nanoleaves solutions on inter-digitated electrode (IDE) silicon substrate test chips were fabricated [35]. After that, annealing process was conducted at 400 °C to remove the attached ligands, OA, and DT, facilitating the carrier transportation among nanoleaves. Shown in Fig. 6, the I-V curve demonstrates the photoresponsive property of thin films composed of CuInS2 nanoleaves. The drop-casting CuInS2 film exhibits observable photoresponsive property, 11-fold increase from 7.2 × 10−8 A at 10 V in the dark to 8.1 × 10−7 A under illumination. The enhancement of photoresponsive current of CuInS2 films in this work is deduced to be beneficial from fast carriers transport for their nature of single crystalline. Tang and Sargent [35] reported that colloidal CuInSe2, CuGaSe2, and Cu(InGa)Se2 nanoparticle-based thick films showed photoresponse, from 2–10-fold current increase compared dark with lamp. And also, ZnO nanocrystal-based field-effect transistors exhibited much improved semiconducting properties with spin-coated ZnO thin-films by tuning the shape from zero-dimensional nanocrystals to one-dimensional nanorods [36]. In contrast to nanocrystals, nanoleaves with one-dimensional morphology do not have grain boundary along their length [12, 36, 37], which might have faster carrier transport than percolation through a random polycrystalline network in nanocrystals, and thus enhancing photoresponse.
Conclusions
We demonstrated the catalyst-assisted growth of wurtzite CuInS2 nanoleaves in solution by using commercial copper nanoparticles as staring materials. The transformation from copper nanoparticle to copper oxide and then copper sulfide Cu1.94S underwent quickly in the presence of oxygen atmosphere at the elevated temperature. Then, Cu1.94S nanocrystals played the catalytic roles for the growth of wurtzite CuInS2 nanoleaves. The 2D-projected elemental maps for three elements demonstrated the evenly distribution of those elements among CuInS2 nanoleaves. Photoresponses of CuInS2 nanoleaves were evaluated by I-V measurements, 11-fold increase compared with that in the dark. The enhancement of photoresponsive current of CuInS2 film is believed to be attributed to one-dimensional single crystalline nature of CuInS2 nanoleaves.
References
S. R. Kodigala. Cu(In1-xGax)Se2 Based thin film solar cells. Academic Press, 35; 2011.
Chang SH, Chiang MY, Chiang CC, Yuan FW, Chen CY, Chiu BC, et al. Facile colloidal synthesis of quinary CuIn1−xGax (SySe1−y)2 (CIGSSe) nanocrystal inks with tunable band gaps for use in low-cost photovoltaics. Energy Environ Sci. 2011;4(12):4929–32.
Xie RG, Rutherford M, Peng XG. Formation of high-quality I-III-VI semiconductor nanocrystals by tuning relative reactivity of cationic precursors. J Am Chem Soc. 2009;131(15):5691–7.
Kolny-Olesiak J, Weller H. Synthesis and application of colloidal CuInS2 semiconductor nanocrystals. ACS Appl Mater Inter. 2013;5(23):12221–37.
Stanbery BJ. Copper indium selenides and related materials for photovoltaic devices. Crit Rev Solid State Mater Sci. 2002;27(2):73–117.
Chen S, Gong X, Walsh A, Wei S-H. Electronic structure and stability of quaternary chalcogenide semiconductors derived from cation cross-substitution of II-VI and I-III-VI2 compounds. Phys Rev B. 2009;79(16):165211.
Pan DC, An LJ, Sun ZM, Hou W, Yang Y, Yang ZZ, et al. Synthesis of Cu-In-S ternary nanocrystals with tunable structure and composition. J Am Chem Soc. 2008;130(17):5620–1.
Kruszynska M, Borchert H, Parisi J, Kolny-Olesiak J. Synthesis and shape control of CuInS2 nanoparticles. J Am Chem Soc. 2010;132(45):15976–86.
Singh A, Geaney H, Laffir F, Ryan KM. Colloidal synthesis of wurtzite Cu2ZnSnS4 nanorods and their perpendicular assembly. J Am Chem Soc. 2012;134(6):2910–3.
Zhong H, Zhou Y, Ye M, He Y, Ye J, He C, et al. Controlled synthesis and optical properties of colloidal ternary chalcogenide CuInS2 nanocrystals. Chem Mater. 2008;20(20):6434–43.
Kuno M. An overview of solution-based semiconductor nanowires: synthesis and optical studies. Phys Chem Chem Phys. 2008;10(5):620–39.
Hochbaum AI, Yang P. Semiconductor nanowires for energy conversion. Chem Rev. 2009;110(1):527–46.
Weber J, Singhal R, Zekri S, Kumar A. One-dimensional nanostructures: fabrication, characterisation and applications. Int Mater Rev. 2008;53(4):235–55.
Law M, Goldberger J, Yang P. Semiconductor nanowires and nanotubes. Annu Rev Mater Res. 2004;34(1):83–122.
Wooten AJ, Werder DJ, Williams DJ, Casson JL, Hollingsworth JA. Solution–liquid–solid growth of ternary Cu–In–Se semiconductor nanowires from multiple- and single-source precursors. J Am Chem Soc. 2009;131(44):16177–88.
Zhang Y, Geng H, Zhou Z, Wu J, Wang Z, Zhang Y, et al. Development of inorganic solar cells by nanotechnology. Nano-Micro Letters. 2012;4(2):124–34.
Cao Y, Wu Z, Ni J, Bhutto WA, Li J, Li S, et al. Type-II core/shell nanowire heterostructures and their photovoltaic applications. Nano-Micro Letters. 2012;4(3):135–41.
Kolasinski K. Catalytic growth of nanowires: vapor-liquid-solid, vapor-solid-solid, solution-liquid-solid and solid-liquid-solid growth. Curr Opin Solid State Mater Sci. 2006;10(3-4):182–91.
Trentler TJ, Hickman KM, Goel SC, Viano AM, Gibbons PC, Buhro WE. Solution-liquid-solid growth of crystalline III-V semiconductors: an analogy to vapor-liquid-solid growth. Science. 1995;270(5243):1791–4.
Hanrath T, Korgel B. Supercritical fluid-liquid-solid (SFLS) synthesis of Si and Ge nanowires seeded by colloidal metal nanocrystals. Adv Mater. 2003;15(5):437–40.
Puthussery J, Kosel T, Kuno M. Facile synthesis and size control of II-VI nanowires using bismuth salts. Small. 2009;5(10):1112–6.
Zhou JC, Huang F, Xu J, Wang YS. Controllable synthesis of metal selenide heterostructures mediated by Ag2Se nanocrystals acting as catalysts. Nanoscale. 2013;5(20):9714–9.
Regulacio MD, Ye C, Lim SH, Bosman M, Polavarapu L, Koh WL, et al. One-pot synthesis of Cu1.94S–CdS and Cu1.94S–ZnxCd1−xS nanodisk heterostructures. J Am Chem Soc. 2011;133(7):2052–5.
Han W, Yi L, Zhao N, Tang A, Gao M, Tang Z. Synthesis and shape-tailoring of copper sulfide/indium sulfide-based nanocrystals. J Am Chem Soc. 2008;130(39):13152–61.
Wang J, Chen K, Gong M, Xu B, Yang Q. Solution–solid–solid mechanism: superionic conductors catalyze nanowire growth. Nano Lett. 2013;13(9):3996–4000.
Wang J, Feng H, Chen K, Fan W, Yang Q. Solution-phase catalytic synthesis, characterization and growth kinetics of Ag2S-CdS matchstick-like heteronanostructures. Dalton Trans. 2014;43(10):3990–8.
Guria AK, Sarkar S, Patra BK, Pradhan N. Efficient superionic conductor catalyst for solid in solution–solid–solid growth of heteronanowires. J Phys Chem Lett. 2014;5(4):732–6.
Li Q, Zhai L, Zou C, Huang X, Zhang L, Yang Y, et al. Wurtzite CuInS2 and CuInxGa1-xS2 nanoribbons: synthesis, optical and photoelectrical properties. Nanoscale. 2013;5(4):1638–48.
Wang S, Yang S. Growth of crystalline Cu2S nanowire arrays on copper surface: effect of copper surface structure, reagent gas composition, and reaction temperature. Chem Mater. 2001;13(12):4794–9.
Wang S, Yang S. Surfactant-assisted growth of crystalline copper sulphide nanowire arrays. Chem Phys Lett. 2000;322(6):567–71.
Wang F, Dong A, Sun J, Tang R, Yu H, Buhro WE. Solution–liquid–solid growth of semiconductor nanowires. Inorg Chem. 2006;45(19):7511–21.
Shen S, Zhang Y, Liu Y, Peng L, Chen X, Wang Q. Manganese-doped Ag2S-ZnS heteronanostructures. Chem Mater. 2012;24(12):2407–13.
Zhuang T-T, Fan F-J, Gong M, Yu S-H. Cu1.94S nanocrystal seed mediated solution-phase growth of unique Cu2S–PbS heteronanostructures. Chem Commun. 2012;48(78):9762–4.
Han S, Gong M, Yao H, Wang Z, Yu S, Controlled O-P. Synthesis of hexagonal-prismatic Cu1.94S-ZnS, Cu1.94S-ZnS-Cu1.94S, and Cu1.94S-ZnS-Cu1.94S-ZnS-Cu1.94S heteronanostructures. Angew Chem Int Ed. 2012;51(26):6365–8.
Tang J, Hinds S, Kelley SO, Sargent EH. Synthesis of colloidal CuGaSe2, CuInSe2, and Cu(InGa)Se2 nanoparticles. Chem Mater. 2008;20(22):6906–10.
Sun B, Sirringhaus H. Solution-processed zinc oxide field-effect transistors based on self-assembly of colloidal nanorods. Nano Lett. 2005;5(12):2408–13.
Steinhagen C, Akhavan VA, Goodfellow BW, Panthani MG, Harris JT, Holmberg VC, et al. Solution–liquid–solid synthesis of CuInSe2 nanowires and their implementation in photovoltaic devices. ACS Appl Mater Inter. 2011;3(5):1781–5.
Acknowledgements
This work was supported by the funds from the NSFC (51102186, 51302194, 21101120, 61471270, 51025207), the NSFZJ (LQ12E02006), and the Research Fund of College Student Innovation of Zhejiang Province (2014R424027).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
CC synthesized the materials, carried out the experiments, with assistance from LZ for the TEM measurements. LZ constructed the experimental setup. ZS carried out XRD measurement. LZ and YY conducted I-V measurements. CZ and SH designed the experiments and wrote the manuscript with the assistance of all other co-authors. All authors read and approved the final manuscript.
Additional file
Additional file 1: Figure S1.
HAADF-STEM images of CuInS2 nanoleaf. (a) the composite STEM-EDS micrograph, (b) EDS spectra collected from head and body parts of nanoleaf. Mo and Si element peaks attributed to molybdenum grid and EDS detector. (c-e) STEM-EDS elemental maps of Cu, In, and S, respectively. The yellow cycles indicates the head part of nanoleaf. Figure S2. HAADF-STEM image of CuInS2 nanoleaf. (a) SE image, (b) EDS line scan profile. The analysis was made from the head part toward the body part of nanoleaf, as indicated by hollow blue arrow. Figure S3. HAADF-STEM image of CuInS2 nanoleaf. (a) SE image, (b) EDS line scan profile. The analysis was made from the head part toward the body part of nanoleaf, as indicated by hollow blue arrow.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cai, C., Zhai, L., Zou, C. et al. Cu1.94S-Assisted Growth of Wurtzite CuInS2 Nanoleaves by In Situ Copper Sulfidation. Nanoscale Res Lett 10, 294 (2015). https://doi.org/10.1186/s11671-015-0996-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11671-015-0996-y