Abstract
Gapped hollow BaTiO3 nanospheres with an apparent diameter of 93 ± 19 nm (shell thickness of 10–20 nm) were synthesized via a dynamic hydrothermal process using TiO2 sols and Ba2+ ions as the Ti and Ba sources in alkaline aqueous solutions. The phases and morphologies of the BaTiO3 samples were characterized by X-ray diffraction (XRD), SEM, TEM, and Raman spectra. The effects of the hydrothermal temperatures and durations, NaOH concentrations, and Ba/Ti ratios on the formation of gapped hollow BaTiO3 nanospheres were systematically investigated. The optimum conditions for forming gapped hollow BaTiO3 nanospheres are hydrothermal treatment at 180 °C for 10–20 h under a continuous magnetic stirring with NaOH concentrations of about 1 mol/L and molar Ba/Ti ratios of 1.2–1.5. The formation mechanism of the gapped hollow BaTiO3 nanospheres is understood as the combination of the orientated attachment and reversed crystal growth.
Similar content being viewed by others
Background
Hollow nanostructures with unique microstructures and functional properties have attracted increasing attention in scientific and engineering aspects [1, 2]. Hollow nanostructures show enhanced functional properties because of their high surface areas [3, 4]. The Kirkendall effect, Ostwald ripening, and hard/soft templates have also been used to construct hollow nanostructures [5–7]. However, the fine control on the formation of hollow nanostructures and deep understanding of the related mechanisms are still a big challenge.
Barium titanate (BaTiO3, BTO), a high-k dielectric material, has been widely applied in commercial multilayer ceramic capacitors [8, 9]. Being a lead-free ferroelectric ceramic, BTO is an environment-friendly material for various applications, including capacitors [10], electronic devices [11, 12], ultrasonic transducer [13], energy storage capacity [14], microwave absorbers [15, 16], and semiconductors [17–19]. Barium titanate is usually of four different phases which depend on the formation temperature: the paraelectric cubic, ferroelectric tetragonal, orthorhombic, and rhombohedral phases [19]. Hollow structures can improve the dielectric properties of BaTiO3 nanoparticles [20, 21]. Some methods have been developed in the past decades to fabricate BaTiO3 hollow nanostructures, such as the layer-by-layer colloidal templating method [21], molten hydrated salt method [22], and doping method [23]. These methods for the synthesis of hollow BaTiO3 nanostructures are complicated, and simpler processes are required.
Wet chemical methods have widely been developed to synthesize nanoscale powders (5–100 nm) with advantages of high purity, near-atomic level homogeneity, and adjustable compositions [24–26]. Among the wet chemical methods, the hydrothermal process is thought as a versatile, low-cost and environment-friendly method to prepare barium titanate nanoparticles with controlled sizes and morphologies [20, 27, 28]. For example, Geng et al. [29] developed a general hydrothermal process using stick-like titania powders, sodium hydroxide, and soluble target ions as the starting materials to achieve MTiO3 (M = Sr, Ba, and Ca) polyhedra. Hollow (bowl-like) BaTiO3 particles can also be synthesized via a hydrothermal process [30]. Although there are reports on the hydrothermal synthesis of barium titanate nanocrystals [20, 28, 31], the influencing factors and formation mechanism of the barium titanate nanocrystals (especially the hollow nanospheres) are still not clear. It is necessary to investigate the fundamental roles of the synthetic parameters (temperatures, precursors, times, etc.) on the crystallization thermodynamics and kinetics of anisotropic growth of barium titanate [26, 32]. Hydrothermal syntheses of BaTiO3 particles with the aid of surfactants (i.e., PEG) are well investigated [28, 33], but these syntheses of hollow BaTiO3 particles without surfactants are seldom reported [30].
In this work, we developed a dynamic hydrothermal process to synthesize gapped hollow BaTiO3 nanospheres without surfactants, just by modifying the common static hydrothermal process [34] with a continuous magnetic stirring during the whole crystallization process (Scheme 1). The effects of hydrothermal temperatures, the molar Ba/Ti ratios, NaOH concentrations, and hydrothermal durations on the formation of gapped hollow BaTiO3 nanospheres are systematically investigated. The morphologies, particle sizes, and phase compositions of the as-obtained BaTiO3 particles are carefully characterized. The relationships between the phases, microstructures, and synthetic conditions are investigated.
Methods
Tetrabutyl titanate (Ti(OC4H9)4, analytically pure) and barium nitrate (Ba(NO3)2, chemically pure) were purchased from Sinopharm Chemical Reagent Co., Ltd.; sodium hydrate (NaOH, analytically pure), glacial acetic acid (C2H4O2, analytically pure), and isopropyl alcohol (C3H8O, analytically pure) were purchased from Tianjing Kermel Chemical Reagent Co., Ltd.; and ethanol (analytically pure) was purchased from Anhui Ante Biochemical Co., Ltd. All reagents were used as received without further purification. Distilled water was used in the experiments.
Gapped hollow BaTiO3 nanospheres were synthesized by treating the mixtures of TiO2 sols and Ba(NO3)2 under a dynamic hydrothermal condition (Scheme 1). For the preparation of TiO2 sols, Ti(OC4H9)4 (80 mL) was mixed with isopropyl alcohol (80 mL) to form a homogeneous solution, which was then added to an acidic solution made by mixing glacial acetic acid (30 mL) and distilled water (800 mL). The above suspension was kept stirring for 3–5 days at room temperature to form a semitransparent TiO2 sol. For the synthesis of gapped hollow BaTiO3 nanospheres, Ba(NO3)2 solids were firstly dissolved to 60 mL of TiO2 sol, and the as-obtained sol was then mixed with a NaOH solution (20 mL). During the above mixtures, the molar Ba/Ti ratios (R Ba/Ti) were kept 1–2, and the NaOH concentrations ([NaOH]) were 1–3 mol/L. The suspension (~80 mL) was then transferred to a 100-mL Teflon-lined stainless steel autoclave with a magnetic stirrer and tightly sealed. Then, the autoclave was placed in oil bath (100–200 °C) under a magnetically stirring condition for 30 min to 50 h. After reaction for given times, the solid samples were collected and washed several times, followed by drying at 120 °C for about 12 h. The detailed experimental conditions for the synthesis of BaTiO3 samples are summarized in Table 1.
The phase compositions of the as-obtained samples were determined using X-ray diffraction (XRD) performed on an XD-3 X-ray diffractometer (Beijing Purkinje General Instrument Co., Ltd., China) with Cu Kα irradiation (λ = 0.15406 nm). The morphologies and microstructures of the samples were observed using a field-emission electron scanning microscope (FE-SEM; JEOL 7500F) and a field-emission transmission electron microscope (FE-TEM; Tecnai G2 F20, accelerating voltage of 200 kV, Philips) with an attachment of energy-dispersive analysis of X-ray (EDAX). Raman spectra were recorded in the 100–1000 cm−1 wavenumber range using a Horiba Xplora Raman microscope (Horiba Scientific).
Results and Discussion
Phases, Compositions, and Morphologies of Gapped Hollow BaTiO3 Nanospheres
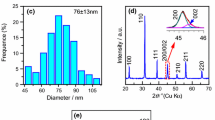
Gapped hollow BaTiO3 nanospheres have been synthesized via a simple dynamic hydrothermal process. Figure 1 shows the SEM observations and XRD pattern of the typical BaTiO3 sample (S1 in Table 1) obtained at 180 °C for 20 h with [NaOH] = 1 mol/L and R Ba/Ti = 1.2. Figure 1a shows a low-magnification SEM image, which suggests that the as-obtained sample consists of well-dispersed nanospheres with uniform morphology and size. The enlarged SEM image in Fig. 1b shows that the sphere-like nanoparticles are of a hollow and gapped microstructure. The apparent particle sizes of the gapped hollow BaTiO3 spheres are 93 ± 19 nm according to the statistical analysis of SEM observations, as shown in Fig. 1c. The shell thickness of the hollow sphere is about 10–20 nm according to the SEM observation (Fig. 1b). The phase composition of the gapped hollow BaTiO3 nanospheres is determined by XRD measurement, and a typical XRD pattern is shown in Fig. 1d. The sample can be indexed to a mixture of cubic and tetragonal BaTiO3 phases [11, 33]. Actually, it is difficult to distinguish the tetragonal BaTiO3 phase from the cubic one just by XRD patterns. If the as-obtained BaTiO3 sample is thought as a cubic BaTiO3 phase (space group, Pm3, a = 4.03 Å, JCPDS card no. 31-0174), the obvious peaks (2θ) at around 22.12°, 31.52°, 38.81°, 45.17°, 50.80°, 56.10°, and 65.76° belong to the (100), (110), (111), (200), (210), (211), and (220) reflections of cubic BaTiO3 phase, respectively. The calculated cell parameter is a = 4.0135(8) Å using a UnitCell program (a method developed by TJB Holland and SAT Redfern) by minimizing the sum of squares of residuals in 2θ. The XRD pattern of S1 is similar to that of the literature result of ref. [33], which is indexed to a cubic BaTiO3 phase. Therefore, it seems safe that the S1 sample mainly consists of cubic BaTiO3 phase.
To confirm the hollow and gapped spherical morphology, we observed the as-obtained BaTiO3 sample using a TEM microscope, and the typical results are shown in Fig. 2. The low-magnification TEM image in Fig. 2a shows that the BaTiO3 particles are uniform, highly dispersed, and semitransparent, seeming of a hollow structure. The high-magnification TEM image in Fig. 2b definitely shows that the BaTiO3 particles are of a hollow and gapped spherical morphology with an apparent diameter of ~100 nm. The thickness of the gapped hollow sphere in Fig. 2c is less than 20 nm. Figure 2d shows the corresponding selected area electron diffraction (SAED) pattern of the BaTiO3 particle (Fig. 2c). One can see that the SAED pattern consists of several sets of diffraction spots, indicating that the gapped hollow BaTiO3 particle is composed of several parts and each of them is of a local single-crystalline structure. The results of TEM observations well agree with the SEM results in Fig. 1. Dai et al. [30] have reported a successful synthesis of bowl-like single-crystalline BaTiO3 nanoparticles via a conventional hydrothermal process using Ba(OH)2·8H2O and TiO2 as precursors. The gapped hollow BaTiO3 nanospheres obtained in the present synthesis are similar to the above bowl-like nanoparticles in morphology although the synthetic process is very much different from each other.
The microstructure of the gapped hollow BaTiO3 nanospheres is also characterized by N2 adsorption-desorption test. Figure 3 shows the typical N2 adsorption-desorption isotherms and pore-size distribution curve. The adsorption isotherm shows an obvious increase at higher relative pressure, indicating that there is a weak interaction between N2 molecules and gapped hollow BaTiO3 nanospheres. Similarly, the desorption isotherm in Fig. 3 shows a sharp decrease with the decrease in the relative pressure. It is noteworthy that the absorption curve does not overlap with the desorption one, suggesting that the N2 molecules are not totally reversible at desorption. The pore-size distribution curve is shown as the inset of Fig. 3, which indicates that there are some large pores with sizes of 250–750 nm. Those large pores probably result from the loose aggregation of the gapped hollow BaTiO3 nanospheres (Figs. 1 and 2). The specific Brunauer-Emmett-Teller (BET) surface area is calculated to be 22.5 m2/g.
Factors Influencing the Formation of Gapped and Hollow BaTiO3 Nanospheres
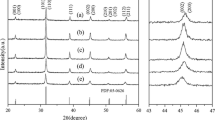
We firstly investigate the effects of NaOH concentrations on the phase composition and morphology of BaTiO3 nanocrystals derived via the dynamic hydrothermal process. From the thermodynamic point of view, BaTiO3 can be easily formed in strong alkaline conditions. To check the effect of NaOH concentrations on the crystallinity and morphology, we change the NaOH concentrations in a range of 1–3 mol/L to synthesize BaTiO3 samples. Figure 4 shows the typical XRD patterns of the BaTiO3 samples synthesized with various NaOH concentrations at 180 °C for 10 h with R Ba/Ti = 1.2. As the XRD patterns in the 2θ range of 20°–70° show (left in Fig. 4), the as-synthesized samples show similar XRD patterns, which can be indexed to BaTiO3 phases (tetragonal (JCPDF no. 05-0626) or cubic (JCPDF no. 31-0174)), and no impure phases can be found [11, 33, 35]. The enlarged XRD patterns in the 2θ range of 44°–46° show that the XRD peak at around 45° becomes wider and wider as the NaOH concentration increases from 1 to 3 mol/L, indicating that the higher NaOH concentration is favorable in forming tetragonal BaTiO3 phase [26, 35].
Figure 5 shows the typical SEM observations of the BaTiO3 samples synthesized with different NaOH concentrations via the dynamic hydrothermal process at 180 °C for 10 h with a Ba/Ti ratio of 1.2. As the SEM images shown in Fig. 5a–e, one can see that low NaOH concentrations (e.g., [NaOH] = 1 mol/L in Fig. 5a) are favorable in forming hollow BaTiO3 nanospheres, whereas the high concentrations (e.g., [NaOH] = 3 mol/L in Fig. 5e) improve the formation of solid BaTiO3 nanospheres. Also, the NaOH concentrations have some effects on the particle sizes of the as-obtained BaTiO3 samples. Figure 5f shows the plot of the particle sizes as function of NaOH concentrations, and their particle sizes were statistically analyzed on the basis of SEM images. One can see that the particle sizes of the as-obtained BaTiO3 samples decrease from about 90 to 60 nm as the NaOH concentrations increase from 1 to 3 mol/L. At the same time, the gapped and hollow structures disappear and a solid spherical nanostructure is formed. One can think that the amount of hydroxyl groups increases as the NaOH concentration increases. In a high [NaOH] solution, the nuclei have more hydroxyl groups on their surfaces, which favor the growth of negatively charged BaTiO3 nanoparticles at a pH ≥10 solution. The negatively charged BaTiO3 nanoparticles can repel each other because of the electrostatic repulsion, which prevents from agglomerating and enhance the dispersibility of the BaTiO3 nanoparticles.
a−e SEM images of the BaTiO3 samples synthesized via a dynamic hydrothermal process at 180 °C for 10 h (R Ba/Ti = 1.2) with various NaOH concentrations: a 1.0 mol/L (S2), b 1.5 mol/L (S3), c 2.0 mol/L (S4), d 2.5 mol/L (S5), and e 3.0 mol/L (S6). f The change of the particle sizes of the BaTiO3 samples as function of NaOH concentrations
We then checked the effects of the Ba/Ti ratios on the formation of gapped hollow BaTiO3 nanospheres in phase compositions and morphologies. We synthesized BaTiO3 samples at 180 °C for 10 h with various Ba/Ti ratios (R Ba/Ti = 1.0–2.0) via the dynamic hydrothermal process in aqueous solutions with [NaOH] = 1 mol/L. Figure 6 shows the XRD patterns of the as-obtained BaTiO3 samples. One can see that the XRD patterns show similar peaks that can be indexed to pure BaTiO3 phase [11, 12]. The enlarged XRD peaks at around 45 °C show that the peaks belonging to {200} deflections become wider and wider as the R Ba/Ti increases from 1 to 2, suggesting that higher R Ba/Ti values favor the formation of tetragonal BaTiO3 phase [11, 35].
The morphologies of the BaTiO3 samples obtained with various Ba/Ti ratios (R Ba/Ti = 1.0–2.0) were observed using a SEM microscope. Figure 7 shows the typical SEM images and the corresponding particle-size distribution plot. Figure 7a shows the SEM image of the BaTiO3 sample obtained with R Ba/Ti = 1, and the sample takes on a spherical morphology. The BaTiO3 spheres are of a size range of 102 ± 21 nm and formed by aggregating small nanoparticles. When the R Ba/Ti increases to 1.2, gapped hollow BaTiO3 nanospheres are obtained, as shown in Fig. 7b. As the R Ba/Ti value continues to increase to 1.5 and 2.0, the BaTiO3 samples obtained show definitely hollow spherical morphologies (Fig. 7c, d). Figure 7e gives the particle-size change of the BaTiO3 samples with the R Ba/Ti values. One can see that the sizes decrease from 102 ± 21 to 57 ± 11 nm as the R Ba/Ti value increases from 1 to 2. The XRD (Fig. 6) and SEM (Fig. 7) results indicate that higher R Ba/Ti values favor the formation of gapped hollow BaTiO3 nanospheres with smaller sizes and more amount of tetragonal phase [22].
We finally investigated the effects of hydrothermal temperatures on the formation of gapped and hollow BaTiO3 nanospheres during the dynamic hydrothermal synthesis. The experiments were done under the same Ba/Ti ratio (R Ba/Ti = 1.2), NaOH concentration ([NaOH] = 1.0 mol/L), and hydrothermal time (10 h), and the hydrothermal temperatures were varied from 100 to 200 °C. Figures 8 and 9 show the typical XRD patterns and SEM observations of the as-obtained BaTiO3 samples. As Fig. 8 shows, all the samples can be indexed to cubic (JCPDF no. 31-0174) or tetragonal (JCPDF no. 05-0626) BaTiO3 phases [12, 36]. The intensity and sharpness of the diffraction peaks increased with the reaction temperatures, indicating a continuous increase of the crystallinity. The widened XRD peaks at around 2θ of 45° possibly from (200) and (002) reflections for the samples obtained at higher temperatures (e.g., 180 and 200 °C) indicate that the higher hydrothermal temperatures are favorable in increasing the amount of tetragonal BaTiO3 phase.
a-d SEM images of BaTiO3 samples synthesized via the dynamic hydrothermal process with R Ba/Ti = 1.2 and [NaOH] = 1.0 mol/L at various temperatures for 10 h: a 100 °C (S10), b 150 °C (S11), c 180 °C (S7), and d 200 °C (S12). e The plot of the particle sizes of the BaTiO3 samples obtained at various temperatures
Figure 9 shows the SEM images (a–d) and particle-size distribution (e) of the BaTiO3 samples synthesized at different hydrothermal temperatures. One can see that the BaTiO3 samples obtained at less than 150 °C consist of spheres formed by aggregating small particles, whereas the sample obtained at 200 °C mostly consists of solid spherical particles. As Fig. 9c shows, the samples obtained at 180 °C show a hollow and gapped spherical morphology. The particle-size plot in Fig. 9e shows that the particle sizes of the samples slightly decrease from 121 ± 30 to 83 ± 20 nm when the hydrothermal temperature increases from 100 to 200 °C.
Taking Figs. 4, 5, 6, 7, 8, and 9 into account, the optimum conditions for the formation of gapped hollow BaTiO3 nanospheres via the present dynamic hydrothermal process are at 180 °C, R Ba/Ti = 1.2−1.5, and [NaOH] = 1.0 mol/L.
Understanding of Growing Gapped Hollow BaTiO3 Nanospheres in Dynamic Hydrothermal Process
To investigate the growth process of gapped hollow BaTiO3 nanospheres, we synthesized a series of BaTiO3 samples with hydrothermal durations varying from 30 min to 30 h and the other conditions were kept the same: R Ba/Ti = 1.2 and [NaOH] = 1.0 mol/L, at 180 °C for 10 h. Figure 10 shows the XRD patterns of the samples obtained. As the XRD patterns in the left column of Fig. 10 show, all the samples with various hydrothermal durations seem to consist of the same BaTiO3 phases [20, 23]. The enlarged XRD pattern at around 2θ = 45° (the right column in Fig. 10) shows that the peak positions did not experience a various shift from lower angles to higher ones as the hydrothermal durations increase from 30 min to 30 h, indicating the structure of the samples obtained is close to the cubic BaTiO3 phase [35]. However, the full width at half maximum (FWHM) values of the XRD patterns at around 2θ = 45° becomes wider and wider; this is due to the fact that these peaks are divided into (002) and (200) peaks. The (200) peak becomes more intense, its 2θ value is higher than the (002) one.
Typical XRD patterns at around 2θ = 20°–70° and 2θ = 44°–46° of the BaTiO3 samples synthesized via the dynamic hydrothermal process with R Ba/Ti = 1.2 and [NaOH] = 1.0 mol/L at 180 °C for various times: a 30 min (S13), b 1 h (S14), c 3 h (S15), d 5 h (S16), e 10 h (S7), f 20 h (S1), and g 30 h (S17)
The position shifting and widening in FWHM of the XRD peaks at around 2θ = 45° suggests that the percentage of the tetragonal phase in the BaTiO3 sample increases with the increase in hydrothermal duration.
Figure 11 shows the typical SEM images of the BaTiO3 samples obtained with various hydrothermal durations (form 10 min to 50 h). At the initial stages, i.e., Fig. 11a, b, the samples consist of solid spheres with about 100 nm in diameter, and the solid spheres are formed by aggregating smaller nanoparticles (5–10 nm). When the hydrothermal times are more than 3 h (e.g., 3–20 h), the BaTiO3 samples obtained tend to form gapped and hollow nanospheres, as shown in Fig. 11c−g. But when the hydrothermal duration is too long, for example, more than 30 h, the BaTiO3 samples tend to form solid nanospheres.
To understand the growth mechanism, we observed a typical BaTiO3 sample obtained at the initial stage with a hydrothermal time of 1 h using the TEM technique. Figure 12 shows the typical TEM observations. The low-magnification TEM image in Fig. 12a shows that the as-obtained BaTiO3 sample with a short hydrothermal duration of 1 h is mainly composed of highly dispersed spherical particles with sizes of 80–120 nm. The enlarged TEM image shown in Fig. 12b indicates that the spherical particles are formed by loosely aggregating small nanoparticles (5–10 nm in size). Figure 12c shows the typical high-resolution TEM image, and the discernible lattice fringe of 0.235 nm (the inset of Fig. 12c) can be indexed to the interplanar distance of {111} planes of the cubic BaTiO3 phase (JCPDS no. 31-0174). Figure 12d shows the corresponding selected area electron diffraction (SAED) pattern, and this ordered pattern can be indexed to single-crystalline cubic BaTiO3 along the zone axis of [1 −1 −1]. The high-resolution TEM image and the corresponding SAED pattern indicate that the spherical particles are of a single-crystalline-like microstructure, suggesting that the nanoparticles are aggregated via an oriented attachment mechanism to form larger nanospheres at the initial stage during the dynamic hydrothermal process [37].
Taking the XRD (Fig. 10), SEM images (Fig. 11), and TEM observations (Fig. 12) into account, we can safely conclude the possible growth paths of the gapped hollow BaTiO3 nanospheres, as shown in Fig. 13. Firstly, TiO2 sols react with Ba2+ ions in a highly alkaline solution to nucleate and form BaTiO3 nanoparticles (BT NPs). Then, the BT NPs are loosely aggregated via an orientated attachment mechanism to form single-crystalline-like nanospheres. Finally, the loosely aggregated single-crystalline-like nanospheres are then crystallized during the dynamic hydrothermal process via the reversed crystal growth mechanism. During the reversed crystal growth, the aggregation of nanoparticles may dominate in the early stages of crystal growth, followed by surface crystallization, and then extension from surface to core, to form gapped hollow nanostructures [32, 36, 38, 39]. The formed gapped hollow spheres with thin shells (10–20 nm in thickness) can be destroyed during the long-term stirring to form some small pieces (Fig. 11h). Those small pieces are resolved and then recrystallize on the pores or edges via the Ostwald ripening process, and finally, solid BaTiO3 particles are again obtained (Fig. 11i). Actually, the BaTiO3 samples obtained under a static hydrothermal process with the same reaction parameters to the dynamic process are composed of solid spherical particles [34]. The continuous stirring seems to favor the surface crystallization and the formation of gapped hollow spheres. It needs to note that different Ti precursors can result in BaTiO3 nanocrystals with various morphologies and microstructures even one uses similar hydrothermal process [40].
We finally further investigated the phase compositions of the BaTiO3 samples using Raman spectroscopy. It is well known that the XRD technique is limited for the crystallographic characterization of fine crystallites owing to extensive broadening of Bragg reflections and generally not very sensitive to transitions involving oxygen displacements. BaTiO3 samples usually have a very small tetragonal distortion (D = (c − a)/a) close to 1 %, and therefore, the angular resolution of peak splitting is limited especially for nanocrystal materials. Raman spectroscopy, a kind of vibrational spectroscopy, is sensitive to the abovementioned distortion. We therefore used Raman spectroscopy to characterize the existence and changes in amount of the tetragonal BaTiO3 component, for understanding the effect of hydrothermal time on the phase composition of BaTiO3 samples obtained with different hydrothermal times. Figure 14 shows the typical Raman spectra of the BaTiO3 samples obtained with hydrothermal times of 10 min, 1 h, 10 h, and 20 h. The bands at 183, 253, and 514 cm−1 arise from crystalline BaTiO3, and they can be observed both in cubic and tetragonal phases, assignable to the scattering from A1(TO) phonons [30]. The tetragonal BaTiO3 phase can be determined by checking the relative intensity of the bands at 306 and 714 cm−1, which are assigned to the B1 and A1(LO) phonons [30]. What is more, the band at 306 cm−1 is assignable to the E(TO) or B1 modes, indicating the asymmetry within the [TiO6] octahedra, while the band at 714 cm−1 is related to the highest-wavenumber longitudinal optical mode (LO) of A1 symmetry [30]. As Fig. 14 shows, the bands at 306 and 714 cm−1 become more and more intense as the hydrothermal time increases from 10 min to 20 h, suggesting that longer hydrothermal time is helpful to enhance the amount of tetragonal BaTiO3 phase. The Raman results agree with the XRD patterns (Fig. 10).
Conclusions
A simple dynamic hydrothermal process has been developed to synthesize hollow and gapped BaTiO3 nanospheres with uniform size (93 ± 19 nm) at 180 °C for 10–20 h in aqueous solutions with [NaOH] = 1.0 mol/L and R Ba/Ti = 1.2. The effects of hydrothermal temperature and time, NaOH concentration, and Ba/Ti ratio on the morphology and phase of the BaTiO3 samples have been investigated systematically on the basis of XRD and SEM results. The optimum conditions for the formation of gapped hollow BaTiO3 nanospheres are determined: [NaOH] = 1.0 mol/L and R Ba/Ti = 1.2, under dynamically hydrothermal treatments at 180 °C for 10–20 h. The forming paths of the gapped hollow BaTiO3 nanospheres can be described as three stages: nucleation (forming nanoparticles), oriented attachment (forming single-crystalline-like spheres), and reversed crystal growth (forming hollow nanospheres with gapped shells). The dynamic hydrothermal process developed here provides an efficient method to obtain highly dispersed BaTiO3 nanospheres with gapped hollow structures, and the gapped hollow BaTiO3 nanospheres are expected to offer unique performance in dialectical applications.
References
Shen L, Song H, Yang G, Wang C. Hollow ball-in-ball Co x Fe3–x O4 nanostructures: high-performance anode materials for lithium-ion battery. ACS Appl Mater Inter. 2015;7:11063–8.
Yu Y, Yin X, Kvit A, Wang X. Evolution of hollow TiO2 nanostructures via the Kirkendall effect driven by cation exchange with enhanced photoelectrochemical performance. Nano Lett. 2014;14:2528–35.
Yang Y, Zhang Q, Fu ZW, Qin D. Transformation of Ag nanocubes into Ag-Au hollow nanostructures with enriched Ag contents to improve SERS activity and chemical stability. ACS Appl Mater Inter. 2014;6:3750–7.
Kim HJ, Jeong HM, Kim TH, Chung JH, Kang YC, Lee JH. Enhanced ethanol sensing characteristics of In2O3-decorated NiO hollow nanostructures via modulation of hole accumulation layers. ACS Appl Mater Inter. 2014;6:18197–204.
Chen Y, Chen HR, Shi JL. Construction of homogenous/heterogeneous hollow mesoporous silica nanostructures by silica-etching chemistry: principles, synthesis, and applications. Acc Chem Res. 2014;47:125–37.
Wang W, Dahl M, Yin Y. Hollow nanocrystals through the nanoscale Kirkendall effect. Chem Mater. 2013;25:1179–89.
Zhang L, Wang H. Interior structural tailoring of Cu2O shell-in-shell nanostructures through multistep Ostwald ripening. J Phys Chem C. 2011;115:18479–85.
Masuda Y, Yamada T, Koumoto K. Synthesis of acicular BaTiO3 particles using acicular barium oxalates. Cryst Growth Des. 2008;8:169–71.
Zou YN, Wu YG, Guo XZ, Tong S, Wang ZY, Zhang L. Effect of particle size on the densification and dielectric properties of BaTiO3 ceramics prepared by liquid phase sintering. Phys Status Solidi A. 2012;209:243–7.
Sonia RK, Patel P, Kumar C, Prakash DK. Temperature synthesis and dielectric, ferroelectric and piezoelectric study of microwave sintered of BaTiO3 ceramics. Ceram Int. 2011;38:1585–9.
Rabuffetti FA, Brutchey RL. Structural evolution of BaTiO3 nanocrystals synthesized at room temperature. J Am Chem Soc. 2012;134:9475–87.
Bocher L, Gloter A, Crassous A, Garcia V, March K, Zobelli A. Atomic and electronic structure of the BaTiO3/Fe interface in multiferroic tunnel junctions. Nano Lett. 2012;12:376–82.
Tang HX, Sodano HA. Ultra high energy density nanocomposite capacitors with fast discharge using Ba0.2Sr0.8TiO3 nanowires. Nano Lett. 2013;13:1373–9.
Arup C. Preparation, characterization and dielectric properties of polyetherimide nanocomposites containing surface-functionalized BaTiO3 nanoparticles. Polym Int. 2012;61:696–702.
Zhu YF, Zhang L, Natsuki T, Fu YQ. Facile synthesis of BaTiO3 nanotubes and their microwave absorption properties. ACS Appl Mat Inter. 2012;4:2101–6.
Zhu YF, Zhang L, Natsuki T, Fu YQ, Ni QQ. Synthesis and microwave absorption properties of electromagnetic functionalized Fe3O4-polyaniline hollow sphere nanocomposites produced by electrostatic self-assembly. Polym Composite. 2013;15:265–9.
Park K, Lee M, Liu Y, Moon S, Hwang GT, Zhu G, et al. Flexible nanocomposite generator made of BaTiO3 nanoparticles and graphitic carbons. Adv Mater. 2012;22:2999–3004.
Anjana S, Surbhi C, Vibha RS, Rohit S, Sahab D. Enhanced photoelectrochemical properties of 100MeV Si8+ ion irradiated barium titanate thin films. J Alloy Compd. 2013;561:114–20.
Liu J, Suna Y, Li ZH. Carbon-modified BiVO4 microtubes embedded with Ag nanoparticles have high photocatalytic activity under visible light. CrystEngComm. 2012;4:7501–8.
Lee JY, Lee JH, Hong SH, Lee YK, Choi JY. Uniform coating of nanometer-scale BaTiO3 layer on spherical Ni particles via hydrothermal conversion of Ti-Hydroxide. J Am Chem Soc. 2005;88:303–7.
Nakano H, Nakamura H. Preparation of hollow BaTiO3 and anatase spheres by the layer-by-layer colloidal templating method. J Am Ceram Soc. 2006;89:1455–7.
Tian XL, Li J, Chen K, Han J, Pan SL. Template-free and scalable synthesis of core-shell and hollow BaTiO3 particles: using molten hydrated salt as a solvent. Cryst Growth Des. 2009;9:4927–32.
Lin MF, Thakur VK, Tan EJ. Dopant induced hollow BaTiO3 nanostructures for application in high performance capacitors. J Mater Chem. 2011;21:16500–4.
Liang WZ, Rao J, Yu A, Shao CL, Zhou XF. Growth dynamics of barium titanate thin films on polycrystalline Ni foils using polymer-assisted deposition technique. ACS Appl Mat Inter. 2012;4:2199–203.
Ju L, Sabergharesou T, Stamplecoskie KG, Hegde M, Wang T. Interplay between size, composition, and phase transition of nanocrystalline Cr3+-doped BaTiO3 as a path to multiferroism in perovskite-type oxides. J Am Chem Soc. 2012;134:1136–46.
Hwang UY, Park HS, Koo KK. Low-temperature synthesis of fully crystallized spherical BaTiO3 particles by the gel–sol method. J Eur Ceram Soc. 2004;87:2168–74.
Hu M, Kurian V, Payzant EA, Rawn CJ, Hunt RD. Wet-chemical synthesis of monodispersed barium titanate particles-hydrothermal conversion of TiO2 microspheres to nanocrystalline BaTiO3. Powder Techinol. 2000;110:2–14.
Chen HJ, Chen YW. Hydrothermal synthesis of barium titanate. Ind Eng Chem Res. 2003;42:473–83.
Yang J, Geng B, Ye Y, Yu X. Stick-like titania precursor route to MTiO3 (M = Sr, Ba, and Ca) polyhedral. CrystEngComm. 2012;14:2959–65.
Deng Z, Dai Y, Chen W, Pei X, Liao J. Synthesis and characterization of bowl-like single-crystalline BaTiO3 nanoparticles. Nanoscale Res Lett. 2010;5:1217–21.
Hou RZ, Ferreira P, Vilarinho PM. Nanoporous BaTiO3 crystallites. Chem Mater. 2009;21:3536–41.
Zhou WZ. Reversed crystal growth: implications for crystal engineering. Adv Mater. 2010;22:3086–92.
Zhan HQ, Yang XF, Wang CM, Chen J, Wen YP. Multiple nucleation and crystal growth of barium titanate. Cryst Growth Des. 2012;12:1247–53.
Gao J, Shi H, Dong H, Zhang R, Chen D. Factors influencing formation of highly-dispersed BaTiO3 nanospheres with uniform sizes in static hydrothermal synthesis. J Nanopart Res. 2015;17(286):1–17.
Joung M-R, Kim J-S, Song M-E, Choi J-H, Nahm S, Choi C-H, et al. Synthesis of highly tetragonal BaTiO3 nanopowders by a two-step alkoxide–hydroxide route. J Alloy Compd. 2011;509:9089–92.
Testino A, Buscaglia MT, Buscaglia V, Viviani V, Bottino C, Nanni P. Kinetics and mechanism of aqueous chemical synthesis of BaTiO3 particles. Chem Mater. 2004;16:1536–43.
Cheng Y, Wang Y, Chen D, Bao F. Evolution of single crystalline dendrites from nanoparticles through oriented attachment. J Phys Chem B. 2005;109:794–8.
Wang WS, Dahl ML, Yin YD. Formation of CdMoO4 porous hollow nanospheres via a self-assembly accompanied with Ostwald ripening process and their photocatalytic performance. Chem Mater. 2013;15:8014–21.
Zhou WZ. Microscopic study of crystal defects enriches our knowledge of materials chemistry. J Mater Chem. 2008;18:5321–5.
Bogicevic C, Thorner G, Karolak F, Haghi-Ashtiani P, Kiat JM. Morphogenesis mechanisms in the solvothermal synthesis of BaTiO3 from titanate nanorods and nanotubes. Nanoscale. 2015;7:3594–603.
Acknowledgements
This work was partly sponsored by the National Natural Science Foundation of China (51172211), China Postdoctoral Science Foundation (2013M531682, 2014T70682), Program for Science & Technology Innovation Talents in Universities of Henan Province (14HASTIT011), Plan for Scientific Innovation Talent of Henan Province (201502), and Special Support Program for High-End Talents of Zhengzhou University (ZDGD13001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing Interests
The authors declare that they have no competing interests.
Authors’ Contributions
JG carried out the synthetic experiments and partly drafted the manuscript. HS, JY, and TL carried out some of the experiments in characterizing the samples, including phase composition and microstructures. RZ participated in the analysis of data. DC conceived of the study, participated in its design and coordination, and drafted and revised the manuscript. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Gao, J., Shi, H., Yang, J. et al. Influencing Factor Investigation on Dynamic Hydrothermal Growth of Gapped Hollow BaTiO3 Nanospheres. Nanoscale Res Lett 10, 329 (2015). https://doi.org/10.1186/s11671-015-1033-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11671-015-1033-x