Abstract
A molecular simulation technique is employed to investigate the transport of H2/CH4 mixture through the two-dimensional (2D) channel between adjacent graphene layers. Pristine graphene membrane (GM) with pore width of 0.515~0.6 nm is found to only allow H2 molecules to enter rather than CH4, forming a molecular sieve. At pore widths of 0.64~1.366 nm, both H2 and CH4 molecules could fill into the GM channel, where the permeability of methane is more preferential than that of hydrogen with the largest CH4/H2 selectivity (1.89) at 0.728 nm. The edge functionalization by –H, –F, –OH, –NH2, and –COOH groups could significantly alter gas permeability by modifying the active surface area of the pore and tuning attractive and/or repulsive interaction with molecules at the entrance of channel. At the pore width of 0.6 nm, the H2 permeability of molecular sieve is enhanced by –H, –F, and –OH groups but restrained by –NH2, especially –COOH with a passing rate of zero. At pore widths of 0.64 and 0.728 nm, both –H and –F edge-functionalized GMs show a preferential selectivity of methane over hydrogen, while the favorable transport for GM–OH is changed from H2 molecules at 0.64 nm to CH4 molecules at 0.728 nm. For GM–NH2, it exhibits an excellent hydrogen molecular sieve at 0.64 nm and then turns into a significant H2/CH4 selectivity at 0.728 nm. Meanwhile, small H2 molecules start to enter the channel of GM–COOH at the pore width up to 0.728 nm. For the largest pore width of 1.336 nm, the influence of edge functionalization becomes small, and a comparable CH4/H2 selectivity is observed for all the considered membranes.
Similar content being viewed by others
Background
In the past few decades, membrane separation technologies exhibit many fascinating properties including low energy consumption, facile operation, and high cost effectiveness and thus have attracted much research attention [1–3]. Among various membrane materials, graphene-based materials have the two-dimensional (2D) carbon sheets with large surface area, chemical stability, mechanical robustness, and high impermeability, and thus, they are considered as one of the most potential classes of separation membranes [4–11]. Because of the high impermeability, the perfect graphene is a good barrier layer for gases and liquids. For molecular separation, therefore, a graphene-based membrane needs to be functionalized with nanopores or nanochannels.
For the porous membranes, the selective molecular permeation could be enabled by opening and controlling the holes on the 2D graphene sheet. In the real-word, however, it is extremely difficult to fabricate a large-area monolayer graphene material with controllable and uniform high-density nanopores. Alternatively, we could prepare a separation membrane with stacked 2D graphene sheets, and the gas molecules could selectively permeate through the 2D channels by controlling the interlayer spacing. Now, the interlayer channel size could be tuned by oxidation [4] and intercalating different-sized cross-linking molecules [12, 13], nanoparticles [14], and nanowires [15].
The 2D channels between two stacked graphene nanosheets may allow the special molecules to pass through but reject the unwanted molecules. For example, Qiu et al. reported that the nanochannels within chemically converted graphene sheets can be controlled by hydrothermal treatment, leading to the selective passing of water and small metal nanoparticles [16]. Nair et al. found that the submicrometer-thick graphene oxide membranes could completely reject liquids, vapors, and gases (He, Ar, H2, and N2), but water is allowed to permeate facilely [17]. They suggested that this seemingly incompatible phenomenon is attributed to a low-friction flow of a monolayer of water through two-dimensional capillaries formed by closely spaced graphene sheets [17]. Recently, Li prepared an ultrathin 1.8-nm-thick graphene oxide membrane by a facile filtration method, which exhibits the ultra-high selectivity for H2/CO2 and H2/N2 mixtures [18]. In addition, the permeability and selectivity of graphene-based membranes to ions and molecules have also been investigated in aqueous media [18].
To explore the mechanism of molecular transport, molecular dynamic (MD) simulation, as a powerful tool, has been employed. For 2D graphene channels, MD simulation showed that the water molecules cannot fill the 2D graphene capillaries with interplanar distance below 0.6 nm, whereas the capillaries with interplanar distance ranging from 0.6 to 1.0 nm could be filled by one and two layers of water molecules [17]. Vieira-Linhares and Seaton studied the transport mechanism of H2/CH4 mixture in the 2D graphite channels by a combination of grand canonical molecular dynamics (GCMC) and dual control volume GCMC (DCV-GCMD) methods [19]. They found the 2D channels show a sieving effect below 0.6 nm, significant selective adsorption of methane between about 0.63 and 1 nm, and poorer separation bigger than 1.0–1.2 nm [19]. Furukawa and Nitta investigated that the permeation of pure and mixed gases (CH4 and C2H6) across carbon membranes with the pore shapes of diamond, zigzag, and straight paths by the nonequilibrium molecular dynamics simulations (NEMD) [20]. Xu et al. performed NEMD simulations on the effect of temperature on the transport and separation of the CO2/CH4 gases through a 2D carbon nanopore [21]. By the NEMD technique, MacElroy and Boyle studied the effect of pressures on the transport of binary H2/CH4 mixtures through a model slit carbon membrane [22]. Recently, Jin et al. reported the flow of methane in the nanochannels of graphite layers at low and high pressures by DCV-GCMD simulations [23].
Theoretical investigations suggested that the nature of the functional groups at the edges of the pores plays a key role in the selective molecular separation for the porous single-layer graphene [6, 24, 25]. Nevertheless, to our best knowledge, the investigation of the influence of the functionalization at the sheet edge on the molecular permeation and separation is rather scarce for the 2D graphene channels. In this work, a molecular simulation technology is used to systematically study the effect of edge functionalization on the H2/CH4 separation through 2D graphene channels with width of 0.515~1.366 nm.
Methods
Molecular models of pristine graphene membrane (GM) and GM with edge modified by functional –H, −F, −OH, −NH2, and –COOH groups were established using Materials Studio [26]. The GM modelled in this work is shown in Fig. 1. The simulation box consists of three basal graphite layers above and below a single isolated pore. Periodic boundary conditions were applied in three dimensions. Therefore, there are six graphite layers between two pores in the x direction, while the membrane is of infinite breadth in the y direction. The length of channel in the z direction is 4.92 nm for pristine GM. The size of simulation box is (1.7 + W) nm × 4.26 nm × 15.028 nm in the x, y, and z directions, respectively, where W is the pore widths of 0.515, 0.6, 0.64, 0.728, 0.855, 1.111, and 1.366 nm. The GM was placed in the middle of the box, and the gas phase and vacuum phase were divided by the fixed GM. The gas phase was the mixture of H2 and CH4 with 1:1 composition and density of 0.03269 g cm−3 at the initial step, similar to the values reported by Tao et al. [27].
MD calculations were performed using the Discover code in Materials Studio of Accelrys Inc. [26]. The interatomic interactions are described by the force field of a condensed-phase optimized molecular potential for atomistic simulation studies (COMPASS) [28]. COMPASS is a first ab initio force field, which has presented a good reliability in describing the absorbate–adsorbent interaction for the gas molecules in carbon-based materials [24, 27]. The cutoff distance for truncation of the intermolecular interactions was set to 1.28 nm, and the Ewald sum technique was used to calculate the electrostatic interaction. The Andersen thermostat method was used to control the temperature of the system at 300 K [29]. MD simulations were carried out in the canonical (NVT) ensemble. During the simulations, the time steps were set to 1 × 107, with a fixed time step of 1 fs. Data was collected every 5 ps.
Electron density was calculated using PBE functional with the double-ξ numerical polarization (DNP) basis set, which was implemented in the DMol3 code in the Materials Studio of Accelrys Inc. [30, 31]. The tolerances of energy, gradient, and displacement convergence were 1 × 10−5 hartree, 2 × 10−2 hartree/nm, and 5 × 10−4 nm, respectively.
The selectivity of component i over component j (S i/j ) is defined by the following schematic equation:
where x i (x j ) is the mole fraction of component i (j) entering the pore and y i (y j ) are the mole fractions of component i (j) in the gas phase.
Results and Discussion
Pristine Graphene Membranes
W = 0.515 − 0.6 nm
The amount of gas molecules entering the channel of GM is shown in Table 1. The final configurations of the gas molecules permeating GMs are given in Additional file 1: Figure S1. Because of a small kinetic diameter of H2 molecule (0.283 nm), 55 molecules are allowed to enter the 2D channel of 0.515 nm at 10 ns (see Table 1). When the pore size increases to 0.6 nm, more H2 molecules (61) can diffuse into the channel. At pore sizes of 0.515 and 0.6 nm, the concentration profile indicates that the stray H2 molecules are distributed throughout two boxes without obvious accumulation in the channel or near the edges of the membrane (Fig. 2b, c), suggesting most of H2 molecules could pass the membrane to the side vacuum boxes with trace amount of adsorbed species in the channel. For CH4 molecules (kinetic diameter of 0.376 nm), they are found to be too big to enter the pores of both 0.515 and 0.6 nm. As shown in Fig. 2b, c, CH4 molecules are found to be restricted to the gas box without molecule distribution in the channel or offside vacuum box. In the gas box, a pronounced peak of adsorbed CH4 is found near the membrane, which is attributed to the strong electrostatic interaction between CH4 and membrane. All these indicate that the membrane with pore size of 0.515~0.6 nm can be as a molecular sieve, where small H2 molecules can pass preferentially, whereas large CH4 is forbidden to penetrate.
W = 0.64 − 1.366 nm
At the pore width of 0.64 nm, CH4 molecules start to enter the channel. At 10 ns, 86 CH4 molecules have diffused into the pore (Table 1). For H2, the amount of entered molecules increases to 74 at 0.64 nm. The corresponding selectivity of CH4 over H2 is found to be 1.39. At the pore width of 0.728 nm, the amounts of entered CH4 and H2 molecules increase to 145 and 77, respectively. The CH4/H2 selectivity also increases to 1.88 at 0.728 nm. However, when the pore size further increases to 0.855, 1.111, and 1.366 nm, the amount of entered CH4 molecules gradually decreases to 129, 121, and 110, respectively, although more and more H2 molecules (80, 82, and 86) diffuse into the channel. Correspondingly, the CH4/H2 selectivity decreases to 1.61, 1.47, and 1.28 for the pores of 0.855, 1.111, and 1.366 nm, respectively. Therefore, it can be seen that with the increasing pore width from 0.515 nm to 1.366 nm, the number of H2 molecules entering the pore increases gradually, whereas a pronounced peak of CH4 is found at 7.28 Å with the maximum CH4/H2 selectivity of 1.89.
Similar to the pores of 0.515 and 0.6 nm, the concentration profiles suggest that most of H2 molecules, which go into the pore, could penetrate the membrane to the offside vacuum boxes at 0.64 − 1.366 nm (Fig. 2d-h). However, CH4 molecules entering the pore prefer to stay in the channel with few molecules passing into the offside vacuum box, especially the channel of 0.728 nm, indicating an optimal CH4-adsorbed pore of 0.728 nm. As a lightweight nonpolar molecule, the main interaction of H2 with graphene sheets is the weak Van der Waals terms. Therefore, H2 molecules could easily pass through the membrane without accumulation in the channel, and the H2 permeability is proportional to the width of the channel (active surface area). For CH4 molecules, the strong electrostatic interaction with graphene sheets results in the packing of methane in the channel.
To further explore the transport mechanism of CH4 in the channels, the density profiles of CH4 across the channel (x axis) as a function of pore width were plotted (Fig. 3). As shown in Fig. 3, at the pores of 0.64, 0.728, and 0.855 nm, a striking peak of CH4 is located at the center of the channels, suggesting that CH4 molecules diffuse in the middle region of the channel via a single layer. When the pore is bigger than 1.1 nm (1.11 and 1.366 nm), the transport of CH4 is found to be near the two surfaces of channel via the double molecular layers, as reflected by two strong peaks of CH4 density profiles. A similar situation was also reported by Vieira-Linhares and Seaton using the DCV-GCMD method [19], where the methane molecules at graphite pore of 0.7 nm are most effectively packed in a single layer, while a double-layer adsorption of methane is found at 2.0 nm. Figure 4b shows the profile of interaction energy between CH4 and graphene surface across the channel (i.e., along x axis). This indicates that a single potential energy well at the center of the pore is located for the channels of 0.64, 0.728, and 0.855 nm, with the deepest well of 0.728 nm (26.2 kJ mol−1), while a double potential energy well is formed in the channels of 1.11 and 1.366 nm and is found to be shallower than that in the 0.64~0.855 nm channels. These explain that the adsorption of CH4 is via the single and double layers in the channels of 0.64~0.855 and 1.11~1.366 nm, respectively, with the optimum adsorption condition of 0.728 nm. In addition, among the pore sizes of 0.64~1.366 nm, 0.728 nm also bears the largest interaction energy with CH4 along the whole z axis of channels (Fig. 4b), which further reflects the maximum CH4 adsorption capacity on the pore of 0.728 nm.
For H2 molecules, the channels show a similar single and double potential energy well at the width of 0.515~0.64 and 0.728~1.366 nm, respectively (see Fig. 4a). However, all the energy wells are very shallow, which are less than 1.09 kJ mol−1. Therefore, H2 molecules are unfavorable to adsorb on the channel surfaces, and the main factor influencing H2 permeability is the active surface area (pore width) rather than the interaction energy.
Edge Functionalization
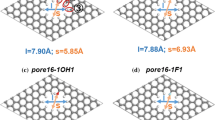
Five functional groups were considered to explore the effect of edge groups on the transport properties of the channel, i.e., hydrogen (–H), fluorine (–F) hydroxyl (–OH), amine (–NH2), and carboxyl (–COOH). The pore widths of 0.6, 0.64, 0.728, and 1.336 nm are selected as models of sieving membrane, monolayer adsorbed membrane, optimum membrane, and bilayer adsorbed membrane, respectively.
W = 0.6 nm
At the pore width of 0.6 nm, we can find the number of H2 molecules entering the channel follows the order GM–F (89) > GM–OH (81) > GM–H (75) > GM (61) > GM–NH3 (47) > GM–COOH (0) (see Table 2). This order may be attributed to the different polarities and sizes of the functional groups. Compared to the pristine edge of graphene, the polar –F, –OH, and –H groups show a stronger intermolecular interaction (Van der Waals terms) with H2 molecules, which could improve the diffusion of H2 into the channel. For the –NH3 and –COOH ligands, they have much larger volume, resulting in a smaller active surface area at the entrance of channel. Therefore, compared to the pristine GM, the permeability of H2 is found to decline for GM–NH3 and even decreases to zero for GM–COOH.
To further understand the effect of edge functionalization on H2 permeability, we calculated the interaction energy between gas molecules and the surface of channel along the z axis. It is found that the edge groups have negligible influence on the interaction with H2 molecules at the center of channel but show a large effect near the entrance of channel (near −2.5 nm along the z axis, see Fig. 5). The edge functionalization by –F, –OH, and –H groups could effectively strengthen the interaction energy with H2 at the entrance of channel, with the sequence of GM–OH > GM–F > GM–H > GM. For GM–NH2 and GM–COOH, however, they form an energy barrier at the entrance, especially GM–COOH ligand, where the energy barrier is much higher than the energetic zero (the energetic sum of free H2 molecule and GM). All these suggest the promotion of H2 transport for –F, –OH, and –H groups but blocking H2 penetration for –NH3, especially –COOH. Note that the contrary order of interaction energy to H2 permeability for GM–F and GM–OH may be attributed to a smaller active surface area of GM–OH than that of GM–F at the entrance, which will be discussed in the following.
W = 0.64 nm
At the pore size of 0.64 nm, both H2 and CH4 molecules could enter the channel of pristine GM. When the edge is functionalized, the amount of H2 molecules entering the pore is enhanced for GM–H (79), GM–F (85), and GM–OH (87), whereas –NH3 and –COOH groups (69 and 0) play a negative role, similar to the situation at 0.6 nm. For the CH4 permeability, only –H and –F groups (90 and 105) play a positive role, while a negative influence is found for –OH, –NH2, and –COOH groups, especially –NH2 and –COOH, which still completely reject CH4 molecules to enter the channel. Therefore, we can find that after edge functionalization, the CH4/H2 separation selectivity for GM–H (1.14) and GM–F (1.23) is comparable with that for GM (1.20), but an inverse CH4/H2 selectivity (0.88) is presented for GM–OH. Furthermore, when the edges of GM are functionalized by the bigger –NH2 and –COOH groups, GM–NH2 becomes an H2 molecular sieve, while GM–COOH remains to completely prohibit the penetration of both H2 and CH4 molecules.
The profiles of interaction energy at 0.64 nm pore indicate that similar to the situation at 0.6 nm, interaction energy with H2 molecules at channel entrance is strengthened after edge functionalization by –F, –OH, and –H groups, while an energy barrier is formed at the entrance of –NH2 and –COOH functionalized membranes but lower than that at 0.6 nm (Fig. 5b). For CH4 molecules, it is interesting that an energy well is formed at the entrance of GM–F, GM–H, and GM (Fig. 5b), which may be caused by the strong electrostatic interaction between CH4 molecules and the polar edge of the membranes. Furthermore, the order of depth of energy well (GM–F > GM–H > GM) is in accordance with that of CH4 permeability. However, because of a steric effect, GM–OH, GM–NH2, and GM–COOH form a high energy barrier at the entrance to restrain the pass of CH4 molecules. Especially, the extra high energy barrier of GM–NH2 and GM–COOH results in a zero probability of CH4 penetration at 0.64 nm.
The permeability of gas molecules is also associated with active surface area of the channel entrance. Therefore, we calculated the electron density isosurface of the entrance for the pristine and edge-functionalized GMs at 6.4 nm (see Fig. 6). As shown in Fig. 6, both –F and –H groups almost have no influence on the pore shape of the entrance, suggesting a comparable active surface area of GM–F and GM–H with pristine GM. For GM–OH, its active surface area is slightly less than that of GM, GM–H, and GM–F. Therefore, –OH group has an inhibiting effect on the permeability of big CH4 molecules, while the influence on small H2 molecules is negligible for the pore of 0.64 nm but relatively large for the small pore of 0.6 nm, as discussed above. After edge functionalization by NH2, the active surface area of GM–NH2 at the entrance becomes apparently small and thus has a negative effect on the H2 permeability and is even too small to allow CH4 molecules to enter. For the biggest –COOH group, it forms an absolutely leak-tight screen to prevent the penetration of both H2 and CH4 molecules.
W = 0.728 nm
When the pore width increases to 0.728 nm, all of the pristine and functionalized GMs (–F, –H, –OH, –NH2, and –COOH) allow H2 molecules to pass. Compared to pristine GM, the amount of H2 molecules entering the channel increases to 81~85 for GM–H, GM–F, GM–OH, and GM–NH2, except GM–COOH, where only 11 H2 molecules could enter. For CH4 molecules, permeability of GM–F, GM–H, and GM–OH is up to maximum (142~145) at 0.728 nm, similar to GM (145). However, only eight CH4 molecules enter the pore of GM–NH2, even there is still no CH4 molecule diffusing into the pore of GM–COOH. As shown in Table 2, the CH4/H2 selectivity for GM–H, GM–F, and GM–OH (1.71 ~ 1.73) is slightly less than that for GM (1.88), while a much low value (0.10) is found for GM–NH2, indicating an inversely high H2 selectivity. In addition, GM–COOH can be as a molecular sieve at pore size of 0.728 nm to allow only H2 to pass.
The interaction energy between gas molecules and the membrane was also calculated at pore size of 0.728 nm (see Fig. 5c). As shown in Fig. 5c, the H2 energy barrier at the entrance of GM–NH2 disappears, according with the comparable permeability of H2 with GM, GM–H, GM–F, and GM–OH. In addition, the height of H2 energy barrier for GM–COOH has become lower than the energetic zero, suggesting the acceptability of H2 transport. For CH4 molecules, both GM–NH2 and GM–COOH form an energy barrier at the entrance of the channel. However, the barrier for GM–NH2 is relatively low and could be overcome by methane molecules, whereas the barrier for GM–COOH is still too high to be overcome.
W = 1.366 nm
At the large pore size of 1.366 nm, the values of H2 and CH4 permeability for both pristine and functionalized membranes fluctuate in a small region of 85~92 and 107~112, respectively, suggesting the influence of edge functionalization is slight for the large pore. As discussed above (see Fig. 4), for both H2 and CH4 molecules, the interaction energy profiles (i.e., along x axis) at 1.366 nm show a double potential well across the channel with location near the surfaces of membranes. Although edge functionalization has some effects on the potential well at the channel entrance (Fig. 5d), the large active surface area may play an important role in gas transport at big 1.336 nm pore and thus results in a comparable gas permeability for all the considered membranes.
Conclusions
The separation of binary H2/CH4 mixture through the 2D graphene channels has been investigated via molecular simulation calculations. The results show that for the pristine GM, the membrane with a pore width of 0.515~0.6 nm can be a molecular sieve, which allows small H2 molecules to enter but forbids large CH4 to pass. Although both H2 and CH4 molecules could transport into the 0.64~1.366 nm channels, a favorable selectivity of methane over hydrogen is observed, with a maximum value of 1.89 at 0.728 nm.
The edge functionalization of GM could modify the active surface area of the pore and tune attractive and/or repulsive interaction with molecules at the entrance of channel, which has a striking influence on the transport of H2/CH4 mixture. (1) At the small pore width of 0.6 nm, the edge modification by –H, −F, and –OH groups could improve the H2 permeability of molecular sieve, but a negative effect is observed for –NH3, especially –COOH, which completely prohibits hydrogen to penetrate. (2) A preferential CH4/H2 selectivity is found for GM–H and GM–F at pore widths of both 0.64 and 0.728 nm, respectively, while the selectivity for GM–OH changes form H2 molecules at 0.64 nm to CH4 molecules at 0.728 nm. For GM–NH2, it always favors transport of H2 at pores between 0.64~0.728 nm, as reflected by an excellent hydrogen molecular sieve property at 0.64 nm and a significant H2/CH4 selectivity at 0.728 nm. With the increasing channel of GM–COOH up to 0.728 nm, gas molecules begin to enter the pore but are restricted to small H2 molecules. When the pore width further increases to 1.336 nm, the influence of edge functionalization becomes weak, resulting in a comparable CH4/H2 selectivity for all the considered membranes.
References
Ockwig NW, Nenoff TM (2007) Membranes for hydrogen separation. Chem Rev 107:4078–110
Li PY, Wang Z, Qiao ZH, Liu YN, Cao XC, Li W, Wang JX, Wang SC (2015) Recent developments in membranes for efficient hydrogen purification. J Membr Sci 495:130–68
Zhao YD, Xie YZ, Liu ZK, Wang XS, Chai Y, Yan F (2014) Two-dimensional material membranes: an emerging platform for controllable mass transport applications. Small 10:4521–42
Huang HB, Yinga YL, Peng XS (2014) Graphene oxide nanosheet: an emerging star material for novel separation membranes. J Mater Chem A 2:13772–82
Xu Q, Xu H, Chen JR, Lv YZ, Dong CB, Sreeprasad TS (2015) Graphene and graphene oxide: advanced membranes for gas separation and water purification. Inorg Chem Front 2:417–24
Huang L, Zhang M, Li C, Shi GQ (2015) Graphene-based membranes for molecular separation. J Phys Chem Lett 6:2806–15
Hu M, Mi BX (2013) Enabling graphene oxide nanosheets as water separation membranes. Environ Sci Technol 47:3715–23
Sun PZ, Zhu M, Wang KL, Zhong ML, Wei JQ, Wu DH, Xu ZP, Zhu HW (2013) Selective ion penetration of graphene oxide membranes. ACS Nano 7:428–37
Kim HW, Yoon HW, Yoon SM, Yoo BM, Ahn BK, Cho YH, Shin HJ, Yang HC, Paik UY, Kwon SG, Choi JY, Park HB (2013) Selective gas transport through few-layered graphene and graphene oxide membranes. Science 342:91–5
Joshi RK, Carbone P, Wang FC, Kravets VG, Su Y, Grigorieva IV, Wu HA, Geim AK, Nair RR (2014) Precise and ultrafast molecular sieving through graphene oxide membranes. Science 343:752–4
Mi BX (2014) Graphene oxide membranes for ionic and molecular sieving. Science 343:740–2
Hung WS, Tsou CH, Guzman MD, An QF, Liu YL, Zhang YM, Hu CC, Lee KR, Lai JY (2014) Cross-linking with diamine monomers to prepare composite graphene oxide-framework membranes with varying d-spacing. Chem Mater 26:2983–90
Xu J, Xing W, Zhao LM, Guo FF, Wu XZ, Xu WB, Yan ZF (2015) The CO2 storage capacity of the intercalated diaminoalkane graphene oxides: a combination of experimental and simulation studies. Nanoscale Res Lett 10:318
Wang WT, Eftekhari E, Zhu GS, Zhang XW, Yan ZF, Li Q (2014) Graphene oxide membranes with tunable permeability due to embedded carbon dots. Chem Commun 50:13089–92
Xu C, Cui A, Xu Y, Fu X (2013) Graphene oxide–TiO2 composite filtration membranes and their potential application for water purification. Carbon 62:465–71
Qiu L, Zhang X, Yang W, Wang Y, Simon GP, Li D (2011) Controllable corrugation of chemically converted graphene sheets in water and potential application for nanofiltration. Chem Commun 47:5810–2
Nair RR, Wu HA, Jayaram PN, Grigorieva IV, Geim AK (2012) Unimpeded permeation of water through helium-leak-tight graphene-based membranes. Science 335:442–4
Li H, Song Z, Zhang X, Huang Y, Li S, Mao Y, Ploehn HJ, Bao Y, Yu M (2013) Ultrathin, molecular-sieving graphene oxide membranes for selective hydrogen separation. Science 342:95–8
Vieira-Linhares AM, Seaton NA (2013) Non-equilibrium molecular dynamics simulation of gas separation in a microporous carbon membrane. Chem Eng Sci 58:4129–36
Furukawa SI, Nitta T (2000) Non-equilibrium molecular dynamics simulation studies on gas permeation across carbon membranes with different pore shape composed of micro-graphite crystallites. J Membrane Sc 178:107–19
Xu LF, Tsotsis TT, Sahimi M (1999) Nonequilibrium molecular dynamics simulation of transport and separation of gases in carbon nanopores. I. Basic results. J Chem Phys 111:3252–64
MacElroy JMD, Boyle MJ (1999) Nonequilibrium molecular dynamics simulation of a model carbon membrane separation of CH4/H2 mixtures. Chem Eng Sci 74:85–97
Jin ZH, Firoozabadi A (2015) Flow of methane in shale nanopores at low and high pressure by molecular dynamics simulations. J Chem Phys 143:104315
Shan MX, Xue QZ, Jing NN, Ling CC, Zhang T, Yan ZF, Zheng JT (2012) Influence of chemical functionalization on the CO2/N2 separation performance of porous graphene membranes. Nanoscale 4:5477–82
Liu HJ, Dai S, Jiang DE (2013) Insights into CO2/N2 separation through nanoporous graphene from molecular dynamics. Nanoscale 5:9984–7
Tildesley DJ, Allen MP. Computer simulation of liquids. Oxford: Clarcndon; 1987.
Tao YH, Xue QZ, Liu ZL, Shan MX, Ling CC, Wu TT, Li XF (2014) Tunable hydrogen separation in porous graphene membrane: first-principle and molecular dynamic simulation. ACS Appl Mater Interfaces 6:8048–58
Sun H (1998) COMPASS: an ab initio force-field optimized for condensed-phase applications—overview with details on alkane and benzene compounds. J Phys Chem B 102:7338–64
Andersen HC (1980) Molecular dynamics simulations at constant pressure and/or temperature. J Chem Phys 72:2384–93
Delley B (2000) From molecules to solids with the Dmol3 approach. J Chem Phys 113:7756–64
Delley B (1996) Fast calculation of electrostatics in crystals and large molecules. J Phys Chem 100:6107–10
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21476264), Distinguished Young Scientist Foundation of Shandong Province (JQ201215), Shandong Provincial Natural Science Foundation (ZR2015BQ009), Promotive Research Fund for Excellent Young and Middle-aged Scientists of Shandong Province (BS2012NJ015), and the Fundamental Research Funds for the Central Universities (12CX02014A and 15CX08010A).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing Interests
The authors declare that they have no competing interests.
Authors’ Contributions
JX and PS contribute equally in this paper and they performed the molecular dynamic simulation and drafted the manuscript together. LZ performed the electron density calculation. ZS and WG plotted and checked the figures. WX gave the final approval of the version to be published. ZY guided the idea and revised and finalized the manuscript. All authors read and approved the final manuscript.
Additional File
Additional file 1:
Supporting information. Fig. S1. Final configurations of the 1:1 H2/CH4 mixture permeating through the 2D channel of pristine and edge-functionalized GMs (DOCX 4515 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Xu, J., Sang, P., Xing, W. et al. Insights into the H2/CH4 Separation Through Two-Dimensional Graphene Channels: Influence of Edge Functionalization. Nanoscale Res Lett 10, 492 (2015). https://doi.org/10.1186/s11671-015-1199-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11671-015-1199-2