Abstract
Two series of nanocrystalline powders of PrCo1 − x Fe x O3 (x = 0.1, 0.3, 0.5, 0.7 and 0.9) of high purity were obtained by sol-gel citrate method at 700 and 800 °C. The formation of continuous solid solution with an orthorhombic perovskite structure (sp. group Pbnm) was observed. A peculiarity of the PrCo1 − x Fe x O3 solid solution is the lattice parameter crossovers, which occurred at certain compositions and revealed in the pseudo-tetragonal or pseudo-cubic metric. An average crystallite size of the PrCo1 − x Fe x O3 samples estimated from the analysis of the angular dependence of the X-ray diffraction (XRD) line broadening varies between 30 and 155 nm, depending on the composition and synthesis temperature.
Similar content being viewed by others
Background
Complex oxides with perovskite structure RMO3, where R and M are rare earth and transition metals, respectively, represent an important class of the functional materials. In particular, the “pure” and mixed rare earth cobaltites and ferrites are used in thermoelectric devices; solid oxide fuel cells, as membranes for partial oxidation of methane; and cleaning oxygen, as catalysts and sensory materials [1–5]. The interest in the rare earth cobaltites RCoO3 is also stimulated by their unique fundamental physical properties, such as different types of magnetic ordering and temperature-induced metal-insulator (MI) transitions conjugated with the spin-state transitions of Co3+ ions [6, 7]. These transitions are strongly affected by the chemical pressure caused by the exchange of cations either in A- or B-sites of perovskite structure [8–10].
Among the mixed rare earth cobaltites-ferrites RCo1 − x Fe x O3, the most extensively studied is a system with La [10–12], whereas information about phase and structural behaviour in the systems with other rare earths is rather limited. Our recent investigations of structural and thermal behaviour of the mixed cobaltites-ferrites with R=Pr, Nd, Sm and Eu obtained by a standard ceramic technique at 1200–1300 °C [13–16] proved a formation of the continuous solid solution with the orthorhombic perovskite structure. In situ high-temperature X-ray synchrotron powder diffraction revealed strong anomalies in the lattice expansion, which are especially pronounced in cobalt-rich specimens. They are reflected in a sigmoidal dependence of the unit cell dimensions, in extra increment of the unit cell volume and in clear maxima of the thermal expansion coefficients [16–19]. These anomalies are related to the changes in spin state of Co3+ ions and conjugated MI transitions. They become less pronounced with the decreasing of the cobalt content in the RCo1 − x Fe x O3 series.
Here, we report the results of structural characterization of nanocrystalline cobaltites-ferrites PrCo1 − x Fe x O3 prepared by sol-gel citrate route.
Methods
Nanocrystalline powders of PrCo1 − x Fe x O3 (x = 0.1, 0.3, 0.5, 0.7 and 0.9) were prepared by sol-gel citrate method. Crystalline Pr(NO3)3·6H2O (99.99 %, Alfa Aesar), Co(NO3)2·6H2O (ACS, Alfa Aesar), Fe(NO3)3·9H2O (ACS, Alfa Aesar) and a citric acid (CC) were dissolved in water and mixed in the molar ratio of n(Pr3+):n(Co2+):n(Fe3+):n(CC) = 1:(1 − х):х:4 according to the PrCo1 − x Fe x O3 nominal compositions. Prepared solutions were gelled at ~90 °C and subsequently treated at the temperatures of 700 and 800 °C for 2 h. Thus, two series of the samples were obtained. Spot-check examination of the cationic composition of the samples was performed by energy dispersive X-ray fluorescence (EDXRF) analysis by using XRF Analyzer Expert 3L.
Laboratory X-ray powder diffraction investigation was performed on the Huber imaging plate Guinier camera G670 (Cu K α1 radiation, λ = 1.54056 Å). The high-resolution X-ray synchrotron powder diffraction examination was performed for the PrCo0.5Fe0.5O3@700 °C and PrCo0.5Fe0.5O3@800°C samples with equiatomic amount of Co and Fe. Corresponding experiments were carried out at the beamline ID22 of ESRF (Grenoble, France) during the beamtime allocated to the Experiment N° MA-2320. All crystallographic calculations were performed by means of the programme package WinCSD [20], which was also used for the evaluation of microstructural parameters of the samples. The average grain size of the powders (D) and lattice strains <ε> = <Δd>/d were estimated from the analysis of angular dependence of the X-ray diffraction (XRD) profile broadening by using the external Si standard for the correction of instrumental broadening. The morphology of the nanoaggregates was investigated by scanning electron microscopy (SEM) by means of an ESEM FEI Quanta 200 FEGi system operated in a low-vacuum mode (70 Pa) and at an acceleration voltage of 15 kV (FEI Company, Eindhoven, NL). The samples were mounted onto conductive carbon tapes adhered on aluminium holders. High-resolution images were obtained using an Everhart-Thornley detector (ETD) for secondary electrons or a solid-state backscattered electron (SSD-BSE) detector.
Results and Discussion
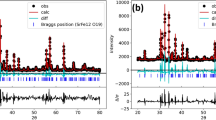
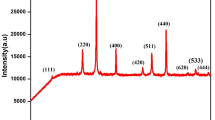
According to X-ray powder diffraction examination of both series of the mixed cobaltites-ferrites PrCo1 − x Fe x O3 obtained at 700 and 800 °C, all the samples synthesized were single phase and possess an orthorhombic perovskite structure isotypic with GdFeO3 (Fig. 1). Only in the iron-rich specimen PrCo0.1Fe0.9O3@800°C the traces of the unidentified parasitic phases could be detected. EDXRF examination of the sample with nominal composition PrCo0.5Fe0.5O3 revealed 70.96(9) wt.% of Pr, 14.98(7) wt.% of Co and 14.06(7) wt.% of Fe, which corresponds to 0.998(2):0.503(3):0.499(3) molar ratio of the metal components.
Full profile Rietveld refinement, performed in space group Pbnm, led to an excellent agreement between calculated and experimental profiles for all PrCo1 − x Fe x O3 samples. In the refinement procedure, the unit cell dimensions and positional and displacement parameters of atoms were refined together with background and peak profile parameters and correction of absorption and instrumental zero shift. No significant difference in the structural parameters was found between two series of the samples.
Precise high-resolution X-ray synchrotron powder diffraction examination confirms phase purity of PrCo0.5Fe0.5O3 samples obtained at 700 and 800 °C. No traces of foreign phases were detected in both samples even applying this very sensitive diffraction technique.
In spite of superb resolution (typical HWFM of the reflections of Si standard are in the limits of 0.003–0.006 2θ o), no reflection splitting was observed at the PrCo0.5Fe0.5O3@700°C and PrCo0.5Fe0.5O3@800°C diffraction patterns due to the rather pseudo-cubic metric of the orthorhombic lattice and essential nanocrystalline size effect on the XRD line broadening.
However, structural parameters of both samples were successfully refined by the full profile Rietveld method in space group Pbnm. As an example, the graphical results of the Rietveld refinement of PrCo0.5Fe0.5O3@800 °C structure are presented in Fig. 2.
Table 1 contains the results of the Rietveld refinement of the PrCo1 − x Fe x O3 samples obtained at 800 °C by using laboratory X-ray and synchrotron powder diffraction data.
Similar to the “pure” PrCoO3 and PrFeO3 compounds, crystal structure of the mixed cobaltites-ferrites PrCo1 − x Fe x O3 can be described as a framework of corner-shared MO6 (M = Co/Fe) octahedra with the Pr atoms occupying holes between them. A projection of the PrCo0.5Fe0.5O3 structure along [001]-direction is shown in Fig. 3.
The analysis of the concentration dependence of the unit cell dimensions of the sol-gel-obtained PrCo1 − x Fe x O3 samples (Fig. 4, solid symbols) proves the formation of continuous solid solution, similar to those observed recently for the mixed praseodymium cobaltites-ferrites obtained by standard ceramic technique (Fig. 4, open symbols) [13, 16]. Peculiarity of the PrCo1 − xFexO3 solid solution is a lattice parameter crossover that occurred at a certain composition, which becomes apparent in the pseudo-tetragonal or pseudo-cubic unit cell dimensions (Fig. 4). The reason for this phenomenon, which was earlier also observed in the related rare earth aluminates, gallates and titanates-chromites [21–26], is the different cell parameter ratios within the same orthorhombic GdFeO3 type of structure observed for the end members of these series.
Concentration dependencies of the normalized unit cell dimensions of PrCo1 − x Fe x O3 series. Solid and open symbols correspond to the samples synthesized by sol-gel technique at 800 °C and by solid-state reactions at 1300 °C, respectively. The dashed lines are guide for the eyes. The lattice parameters of the orthorhombic cell are normalized to the perovskite one as follows: a p = a o /√2, b p = b o /√2, c p = c o /2, V p = V o /4
Microstructural parameters of two PrCo1 − x Fe x O3 series synthesized at 700 and 800 °C were evaluated from the analysis of the XRD profile broadening by using the external Si standard. Average grain size D in both series is estimated to vary within the limit of 30–155 nm, depending on the composition and synthesis temperature (Fig. 5). The D values in the PrCo1 − x Fe x O3@700°C samples systematically decrease with the increasing of iron content, whereas in the PrCo1 − x Fe x O3@800°C series, this parameter goes through the maximum at x = 0.5. In both series, the increase of the lattice strains is observed with increasing iron content (Fig. 5).
Scanning electron microscopy investigation of the PrCo0.5Fe0.5O3 sample prepared at 800 °C (Fig. 6) revealed a lacy morphology of the powder consisting of irregular shaped 60–100-nm nanoparticles.
Conclusions
Two series of the nanocrystalline mixed cobaltites-ferrites PrCo1 − x Fe x O3 (x = 0.1, 0.3, 0.5, 0.7 and 0.9) of high phase purity were prepared by sol-gel citrate method at 700 and 800 °C. The average grain size of the powders estimated from the analysis of angular dependence of the XRD line broadening varies between 30 and 155 nm, depending on the composition and synthesis temperature. Refined structural parameters of the PrCo1 − x Fe x O3@700 °C and PrCo1 − x Fe x O3@800 °C series prove the formation of continuous solid solution as it was shown earlier for the similar series obtained by the standard ceramic technique at 1300 °C. In comparison with a traditional energy- and time-consuming high-temperature solid-state synthesis technique, the low-temperature sol-gel citrate method is a very promising tool for the obtaining of fine powders of the mixed perovskite oxide materials, free of contamination of constituent metal oxides or other parasitic phases.
References
Uhlenbruck S, Tietz F (2004) High temperature thermal expansion and conductivity of cobaltites: potentials for adaptation of the thermal expansion to the demands for SOFCs. Mater Sci Eng B 107:277–282
Mawdsley JR, Krause TR (2008) Rare earth-first-row transition metal perovskites as catalysts for the autothermal reforming of hydrocarbon fuels to generate hydrogen. Appl Catal 334:311–320
Fergus JW (2007) Perovskite oxides for semiconductor-based gas sensors. Sensor Actuator B 123:1169–1179
Itagaki Y, Mori M, Hosoya Y, Aono H, Sadaoka Y (2007) O3 and NO2 sensing properties of SmFe1−x Co x O3 perovskite oxides. Sensor Actuator B 122:315–320
Wu Y, Dujardin C, Granger P, Tiseanu C, Sandu S, Kuncser V, Parvulescu V (2013) Spectroscopic investigation of iron substitution in EuCoO3: related impact on the catalytic properties in the high-temperature N2O decomposition. J Phys Chem C 117:13989–13999
Knizek K, Jirak Z, Hejtmanek J, Veverka M, Marysko M, Maris G, Palstra TTM (2005) Structural anomalies associated with electronic and spin transitions in LnCoO3. Eur Phys J B 47:213–220
Berggold K, Kriener M, Becker P, Benomar M, Reuther M, Zobel C, Lorenz T (2008) Anomalous expansion and phonon damping due to the Co spin-state transition in RCoO3, (R = La, Pr, Nd, and Eu). Phys Rev B 78:1–15
Itoh M, Hashimoto J (2000) Spin state in perovskite cobalt oxides La1−xNdxCoO3. Phys C 341–348:2141–2142
Baier J, Jodlauk S, Kriener M, Reichl A, Zobel C, Kierspel H, Freimuth A, Lorenz T (2005) Spin-state transition and metal-insulator transition in La1−x Eu x CoO3. Phys Rev B 71:014443–10
Karpinsky D, Troyanchuk I, Bärner K, Szymczak H, Tovar M (2005) Crystal structure and magnetic ordering of the LaCo1−x Fe x O3 system. J Phys Condens Matter 17:7219–7226
Nagamoto H, Mochida I, Kagotani K, Inoue H (1993) Change of thermal expansion coefficient and electrical conductivity of LaCo1−x M xO3 (M = Fe, Ni). J Mater Res 8:3158–3162
Ivanova S, Senyshyn A, Zhecheva E, Tenchev K, Stoyanova R, Fuess H (2010) Crystal structure, microstructure and reducibility of LaNi x Co1−x O3 and LaFe x Co −x O3 perovskites. J Solid State Chem 183:940–950
Kharko O, Vasylechko L (2012) Structural behaviour of solid solutions in the PrCoO3–PrFeO3 system. Electronics 734:119–126, Visnyk of Lviv Polytechnic National University
Pekinchak О, Vasylechko L (2014) Crystal structure of new mixed cobaltites-ferrites NdCo1 - x Fe x O3. Electronics 798:34–40, Visnyk of Lviv Polytechnic National University
Kharko O, Vasylechko L, Ubizskii S, Pashuk A, Prots Yu. Structural behaviour of continuous solid solution SmCo −x Fe x O3. Functional Mater. 2014;21, № 2:226–232.
Pekinchak O, Vasylechko L, Berezovets V, Prots Y (2015) Structural behaviour of EuCoO3 and mixed cobaltites-ferrites EuCo1−x Fe x O3. Solid State Phenom 230:31–38
Vasylechko L, Kharko O, Myakush O, Bell A. Spin-state transitions in new mixed RE cobaltites, cobaltites-chromites and cobaltites-ferrites probed by high-temperature lattice expansion. Photon Science—HASYLAB Annual Report. 2012. http://photon-science.desy.de/annual_report/files/2012/20122322.pdf
Kharko O, Vasylechko L (2013) Anomalous thermal expansion of new mixed praseodymium cobaltites-ferrites. Electronics 764:63–69, Visnyk of Lviv Polytechnic National University
Kharko O, Vasylechko L, Prots Y (2013) Сrystal structure and anomalous lattice expansion of new mixed cobaltites-ferrites RCo1−x Fe x O 3 (R = Pr, Nd, Sm, Eu). XII International Conference on Crystal Chemistry of Intermetallic Compounds, Lviv, Ukraine, p 144
Akselrud L, Grin Y (2014) WinCSD: software package for crystallographic calculations (version 4). J Appl Crystallogr 47:803–805
Vasylechko L, Senyshyn A, Bismayer U (2009) Perovskite-type aluminates and gallates. In: Gschneidner KA Jr, Bünzli J-CG, Pecharsky VK (eds) Handbook on the Physics and Chemistry of Rare Earths, vol 39. North-Holland, Netherlands, pp 113–295
Vasylechko L, Shmanko H, Ohon N, Yu P, Hoffmann S, Ubizskii S (2013) Lattice crossover and phase transitions in NdAlO3–GdAlO3 system. J Solid State Chem 198:101–107
Ohon N, Vasylechko L, Prots Yu, Schmidt M. Phase and structural behaviour of SmAlO3–RAlO3 (R = Eu, Gd) systems. Mater Res Bull. 2014;50:509–513.
Vasylechko L, Berkowski M, Matkovskii A, Piekarczyk W, Savytskii D (2000) Structure peculiarities of the La1 - x Nd x GaO3 solid solutions. J Alloys Compd 300–301:471–474
Berkowski M, Fink-Finowicki J, Byszewski P, Diduszko R, Kowalska E, Aleksijko R et al (2001) Czochralski growth and structural investigations of La1−x Pr x GaO3 solid solution single crystals. J Crystal Growth 222:194–201
Vashook V, Vasylechko L, Trofimenko N, Kuznecov M, Otchik P, Zosel J, Guth U (2006) A-site deficient perovskite-type compounds in the ternary CaTiO3–LaCrO3–La2/3TiO3 system. J Alloys Compd 419:271–280
Acknowledgements
The work was supported in parts by the Ukrainian Ministry of Education and Sciences (Project “KMON”) and ICDD Grant-in-Aid programme. The authors thank A. Fitch for the kindly assistance with high-resolution synchrotron powder diffraction measurements at ID22 of ESRF during the beamtime allocated to the Experiment MA-2320. The authors thank N. Koval for the EDXRF analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing Interests
The authors declare that they have no competing interests.
Authors’ Contributions
OP evaluated the XRD data and wrote the manuscript. LV performed structural characterization of the samples and contributed to the manuscript writing. IL and YV performed the sol-gel synthesis of the samples. YP contributed to the X-ray and synchrotron diffraction measurements. WCC performed the scanning electron microscopy measurements. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Pekinchak, O., Vasylechko, L., Lutsyuk, I. et al. Sol-Gel-Prepared Nanoparticles of Mixed Praseodymium Cobaltites-Ferrites. Nanoscale Res Lett 11, 75 (2016). https://doi.org/10.1186/s11671-016-1295-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11671-016-1295-y