Abstract
Water electrolysis is an efficient way for hydrogen production. Finding efficient, cheap, and eco-friendly electrocatalysts is essential to the development of this technology. In the work, we present a first-principles study on the effects of tension on the hydrogen evolution reaction of a novel electrocatalyst, vanadium disulfide (VS2) monolayer. Two electrocatalytic processes, individual and collective processes, are investigated. We show that the catalytic ability of VS2 monolayer at higher hydrogen coverage can be efficiently improved by escalating tension. We find that the individual process is easier to occur in a wide range of hydrogen coverage and the collective process is possible at a certain hydrogen coverage under the same tension. The best hydrogen evolution reaction with near-zero Gibbs free energy can be achieved by tuning tension. We further show that the change of catalytic activity with tension and hydrogen coverage is induced by the change of free carrier density around the Fermi level, that is, higher carrier density, better catalytic performance. It is expected that tension can be a simple way to improve the catalytic activity, leading to the design of novel electrocatalysts for efficient hydrogen production from water electrolysis.
Similar content being viewed by others
Background
Hydrogen, as an important energy carrier, is considered as a replacement of fossil fuels because of its abundant, clean, and renewable characteristics [1, 2]. Hydrogen production can be realized by photo-driven or electrical-driven water splitting [3–7], stream reforming [8], natural gas oxidization [9], carbonation [10], biomass electrolysis [11, 12], etc. Among these methods, catalytic water-spitting is highly appreciated because it is the cleanest way for hydrogen production, where hydrogen is evolving from proton to gas molecule by reduction [3–7]. Especially, electrical-driven water electrolysis has attracted increasing interests because of its high efficiency and eco-friendliness [7, 13–15]. To improve production efficiency and reduce cost, the design and fabrication of highly active, stable, and cheap electrocatalysts are critical to the development of green energy technology. It is well-known that noble metals and their alloys, such as platinum, are the most efficient catalysts in water electrolysis [16–22]. However, large-scale application of these catalysts in hydrogen evolution reaction (HER) for hydrogen production is difficult because they are rare and expensive. Alternatively, finding novel, environmental-friendly electrocatalysts with earth-abundant elements has been carried out with considerable efforts [7, 15].
Recently, transition metal dichalcogenides (TMDs) nanomaterials have been widely investigated as electrocatalysts in water electrolysis [23–32]. Particularly, The TMD monolayers with the formula of MX2, which has a sandwiched structure with one M-atom (M = transition metal element) layer enclosed within two X layers (X = chalcogen element), and the atoms in layers are hexagonally packed, have attracted increasing attention because of their high surface area, active edges states, and high mechanic flexibility [15, 25, 30–40]. For example, MX2 nanoribbons show high HER performance in water electrolysis due to their metallic edges [31], which can be further improved by edge doping and substrate [41, 42]. Metallic WS2 monolayers showed better HER performance than semiconducting counterpart [38]. Recently, Pan reported that VS2 monolayer shows excellent HER activity [32]. However, its performance is reduced at high hydrogen coverage because of reduced conductivity. To improve the HER performance of TMDs monolayers, the basic principle for the design of catalysts is to achieve a near-zero overpotential [18]. It has been reported that the improvement of HER activity can be achieved by doping, forming defect, and hybridizing with graphene [15, 23–42]. Voiry et al. [38] and Lee et al. [43] reported that mechanic strain can efficiently improve the HER activity of WS2 and MoS2 monolayers. To date, the effect of strain on the HER performance of TMDs monolayers at various hydrogen coverages and strength of tension has not been systematically studied. In this work, we carry out first-principles study on the improvement of the HER performance of VS2 monolayer with tension. We show that the improvement of catalytic activity of VS2 monolayer strongly depends to the strength of applied tension, hydrogen coverage, and reaction process. We find that the catalytic performance at higher hydrogen coverage can be enhanced by increasing tension, and both individual/collective processes can happen at the same tension depending on hydrogen coverage. We further show that the catalytic performance is closely related to free carrier density, which is controlled by the tension and hydrogen coverage.
Methods
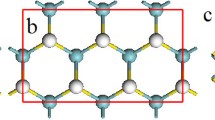
First-principles calculations on the basis of the density functional theory (DFT) [44] and the Perdew-Burke-Eznerhof generalized gradient approximation (PBE-GGA) [45] are carried out to study the effect of tension on the HER activity of VS2 monolayer. The Vienna ab initio simulation package (VASP) [46] with projector augmented wave (PAW) scheme [47, 48] is used in our calculations. The Monkhorst and Pack scheme of k point sampling (3 × 3 × 1) is used for integration over the first Brillouin zone [49] and the cut-off energy is 450 eV. A sufficiently large supercell with 4 × 4 × 1 unit cells and a vacuum region of at least 20 Å in the vertical direction is used to investigate the hydrogen-coverage-dependent HER performance and avoid the interaction between images in neighboring cells (Fig. 1). Good convergence is obtained with these parameters and the total energy is converged to 2.0 × 10−5 eV/atom.
Results and Discussion
The larger supercell with 4 × 4 × 1 unit cells is constructed based on the unit of VS2 monolayer with one surface fully covered by hydrogen atoms, that is, one S atom is bonded with one H atom on one side of the monolayer [50]. The hexagonal supercell with a lattice constant of 13.1 Å (a0) has 16 H, 32 S, and 16 V atoms (Fig. 1). To investigate the effect of tension on its HER performance, the lattice is statically extended from a 0 to a by biaxial stretching or tension \( \left(\varepsilon =\frac{a-{a}_0}{a_0}\times 100\ \%\right) \). The calculation on H-coverage-dependent HER performance is carried out by removing hydrogen atoms one by one from the surface. The H-coverage is defined as \( \frac{n}{16} \) (n = 0 ~ 16). On the basis of the Sabatier principle, the HER performance of electrocatalyst in water electrolysis can be quantified by calculating the reaction free energy of hydrogen adsorption (ΔGH) [18, 19, 32, 33, 38], which can be obtained from following formula:
where ΔE H is the H-coverage-dependent hydrogen chemisorption energy. It can be differential chemisorption energy as calculated by:
or it can be average chemisorption energy calculated by
where n is the number of H atoms adsorbed on a VS2 monolayer under tension. The H-coverage-dependent ΔGH can be obtained by changing n. E(VS2 + nH), E(VS2), and E(H2) in Eqs. (2 and 3) are the energies of monolayer with hydrogen atoms (n), pure VS2 monolayer, and hydrogen molecule, respectively. ΔS H is the difference in entropy. ΔE ZPE is the difference in zero point energy between the adsorbed and the gas phase. ΔE ZPE − TΔS H is about 0.24 eV [18, 19]. Therefore, Eq. (1) can be simplified to ΔG H = ΔE H + 0.24. According to the two methods (Eqs. 2 and 3) for the calculation of hydrogen chemisorption energy, the reaction free energies can be defined as differential ΔGH (d-ΔGH) and average ΔGH (a-ΔGH), which can be used to express the hydrogen production in the individual and collective processes, respectively. The individual process describes that hydrogen is produced one by one, while the collective process shows that all of hydrogen atoms on the surface are simultaneously converted to molecules. It is required that electrocatalyst with optimal HER performance should have a ΔGH near 0 eV.
Our first-principles calculations show that ΔGH is dependent on the H-coverage and can be efficiently tuned by applied tension (Fig. 2). We see that the trend of d-ΔGH as a function of hydrogen coverage under tension is almost similar to that without tension in the individual process (ΔE H is calculated from Eq. 2), that is, d-ΔGH increases as hydrogen coverage increases [32]. Importantly, d-ΔGH is reduced upon the tension applied and the HER activity of VS2 monolayer in individual process at certain hydrogen coverage is improved by tension. The VS2 monolayer under ɛ = 2 % shows best HER performance in individual process with d-ΔGH equal to −0.043 and −0.029 eV at the hydrogen coverages of \( \frac{2}{16} \) and \( \frac{3}{16} \), respectively (Fig. 2a). Under ɛ = 6 %, the best HER performance occurs at the hydrogen coverages of \( \frac{4}{16} \) and \( \frac{5}{16} \) with d-ΔGH equal to 0.023 and 0.018 eV, respectively (Fig. 2a). Further increasing ɛ to 10 %, the VS2 monolayer shows best HER activity at the hydrogen coverages of \( \frac{6}{16} \), \( \frac{7}{16} \), and \( \frac{9}{16} \) (d-ΔGH = 0.024, 0.040, and −0.027 eV) (Fig. 2a). Clearly, the HER activity of VS2 monolayer in individual process is improved at relatively high H-coverage as tension increases, while that at low H-coverage is weakened. At the same time, we see that the effect of tension on the HER activity at full H-coverage \( \left(\frac{16}{16}\right) \) is minor in the individual process (Fig. 2a), because removing one hydrogen atom is subjected to the strong attraction from the remained hydrogen atoms. Compared to the individual process, a-ΔGH in the collective process (ΔE H is calculated from Eq. 3) increases smoothly as the H-coverage increases (Fig. 2b). The best HER activity of VS2 monolayer with a-ΔGH near zero (−0.007 eV) occurs at a hydrogen coverage of \( \frac{5}{16} \) under ɛ = 2 %. Under ɛ = 6 %, the optimal performance (a-ΔGH = −0.005~0.02 eV) is within a H-coverage range from \( \frac{8}{16} \) to \( \frac{10}{16} \). Further increasing tension to 10 %, VS2 monolayer is more active at a H-coverage of around \( \frac{14}{16} \) (a-ΔGH = −0.016 eV). Interestingly, ΔGH in the collective process at full hydrogen coverage is significantly reduced upon the tension applied, which is about 0.1 eV/H at ɛ = 10 % (Fig. 2b). Although the absolute value of a-ΔGH in the collective process is smaller than that in the individual process at full H-coverage, it should be difficult to happen if considering the total number of H atoms. By comparing the individual and collective processes (Fig. 2), we see that individual process is easier than collective process because absolute value of d-ΔGH is smaller than that of a-ΔGH multiplied by the number of H atoms on the surface of the monolayer. However, the collective process prefers to occur at certain H-coverage, where a-ΔGH is close to zero (Fig. 2b). For example, if the VS2 monolayer is fully covered by hydrogen atoms \( \left(\frac{16}{16}\right) \) initially at ɛ = 6 %, the individual process starts first. When the H-coverage is reduced to \( \frac{9}{16} \) (a-ΔGH = 0.003 eV), the collective process occurs because the total reaction energy in collective process (a-ΔGH × 9 = 0.027 eV) is less than d-ΔGH in individual process (0.07 eV). If the H-coverage on the VS2 monolayer is less than \( \left(\frac{8}{16}\right) \), initially, only individual process will happen. Similarly, the collective processes under ɛ = 2 and 10 % are more easier than individual processes at hydrogen coverages of \( \frac{5}{16} \) and \( \frac{14}{16} \), respectively. Generally, the strained VS2 monolayer shows better catalytic performance than other TMDs monolayers and nanoribbons because of the near-zero Gibbs free energy in a wide range of hydrogen coverage.
To reveal the origin of H-coverage-dependent HER activity under tension, the density of state (DOS) is calculated. The carrier density can be estimated from the calculated DOSs. We see that the carrier density changes with the tension and hydrogen coverage (Fig. 3), which may indicate their effects on the HER activity in individual/collective process. At ɛ = 2 %, the VS2 monolayer shows strong metallic characteristic with high free carrier density under the Fermi level at a hydrogen coverage of \( \frac{3}{16} \), as indicated by the continuous DOS spectrum from −3 to 2.2 eV (Fig. 3a). With the increase of hydrogen coverage to \( \frac{9}{16} \), the DOS spectrum separates into two regions with a small gap from −0.9 to −0.5 eV, and the free carrier density under the Fermi level is accordingly reduced (Fig. 3b). Further increasing hydrogen coverage to \( \frac{16}{16} \), the DOS spectrum separates into three regions and the Fermi level is within the band gap, resulting in semiconducting characteristic and poor free carrier density. The reduction of carrier density with the increment of hydrogen coverage should be responsible for the reduction of HER performance (Fig. 2a). At the same time, we see that the DOS spectrum of VS2 monolayer with a hydrogen coverage of \( \frac{3}{16} \) under ɛ = 6 % is divided into two regions and the carrier density under the Fermi level is less than that under ɛ = 2 % (Fig. 3d), leading to the reduction of HER activity. Further increasing the tension (ɛ = 10 %), the gap between two DOS regions is enlarged and the area under the Fermi level is reduced, indicating reduced carrier density and resulting in further reduction of HER activity, which is consistent with the calculated Gibbs free energies (Fig. 2a). Comparing the DOSs of VS2 monolayers with a hydrogen coverage of 9/16 under ɛ = 2, 6, and 10 %, we see that the carrier density under the Fermi level increases as the tension increases (Figs. 3b, e, h), as indicated by the reduced/demolished gap around −0.5 eV, which is also consistent with the calculated Gibbs free energies (Fig. 2a). At full hydrogen coverage, we see that the HER activity of VS2 monolayer is not significantly improved (Fig. 2a). The calculated DOSs show that the Fermi levels are within conduction bands (Fig. 3f, i). However, the strong sharp DOS peaks around −0.8 eV may indicate localized states, which may reduce the carrier density and increase charge recombination centers, leading to differentially removing hydrogen difficult (Fig. 2a).
Conclusions
We carry out first-principles to investigate the effects of tension on the hydrogen evolution reduction of VS2 monolayer. We find that tension can tune the HER performance of VS2 monolayer at different hydrogen coverage. The HER activity at high hydrogen coverage is greatly improved for both of individual and collective processes by increased tension. The hydrogen coverages for best HER performance in both individual and collective processes increase with the increment of tension. Generally, individual process is easier to occur in a wide range of hydrogen coverage, and collective process can happen at certain hydrogen coverage at the same tension. The optimal HER performance of VS2 monolayer with near-zero Gibbs free energy, comparable to Pt, can be achieved by tuning tension. We further show that the change of HER performance at different hydrogen coverages and under various tensions is closely related to the carrier density. High free carrier density is responsible for the improved HER activity. During revision, we noticed that Li et al. recently reported that strain can enhance the catalytic activity of MoS2 basal planes [51]. We see that the tension may provide an efficient way to modify the HER performance of VS2 and other 2D monolayers and find applications to water electrolysis for hydrogen production.
References
Cipriani G, Di Dio V, Genduso F, La Cascia D, Liga R, Miceli R, Galluzzo GR (2014) Perspective on hydrogen energy carrier and its automotive applications. Int J Hydrogen Energy 39:8482–8494
Andrews J, Shabani B (2014) The role of hydrogen in a global sustainable energy strategy. Willy Interdisciplinary Rev Energy Environ 3:474–489
Hisatomi T, Kubota J, Domen K (2014) Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem Soc Rev 43:7520–7535
Peng XN, He C, Fan X, Liu QY, Zhang J, Wang H (2014) Photovoltaic devices in hydrogen production. Int J Hydrogen Energy 39:14166–14171
Christopher K, Dimitrios R (2012) A review on energy comparison of hydrogen production methods from renewable energy sources. Energy Environ Sci 5:6640–6651
Vedarajan R, Ikeda S, Matsumi N (2014) Electrochemical characterization of TiO2/WOx nanotubes for photocatalytic application. Nanoscale Res Lett 9:573
Chen WF, Muckerman JT, Fujita E (2013) Recent developments in transition metal carbides and nitrides as hydrogen evolution electrocatalysts. Chem Commun 49:8896–8909
Contreras JL, Salmones J, Colin-Luna JA, Nuno L, Quintana B, Cordova I, Zeifert B, Tapia C, Fuentes GA (2014) Catalysts for H2 production using the ethanol steam reforming: a review. Int J Hydrogen Energy 39:18835–18853
Dou BL, Song YC, Wang C, Chen HS, Yang MJ, Xu YJ (2013) Hydrogen production by enhanced-sorption chemical looping steam reforming of glycerol in moving-bed reactors. Appl Energy 130:342–349
Sun RY, Li YJ, Zhao JL, Liu CT, Lu CM (2013) CO2 capture using carbide slag modified by propionic acid in calcium looping process for hydrogen production. Int J Hydrogen Energy 38:13655–13663
Tavangar A, Tan B, Venkatakrishnan K (2013) Sustainable approach toward synthesis of green functional carbonaceous 3-D micro/nanostructures from biomass. Nanoscale Res Lett 8:348
Lu XH, Xie SL, Yang H, Tong YX, Ji HB (2014) Photoelectrochemical hydrogen production from biomass derivatives and water. Chem Rev Soc 43:7581–7593
Carmo M, Fritz DL, Merge J, Stolten D (2013) A comprehensive review on PEM water electrolysis. Int J Hydrogen Energy 38:4901–4934
Yuan XX, Hu XX, Ding XL, Kong HC, Sha HD, Lin H, Wen W, Shen GX, Guo Z, Ma ZF, Yang Y (2013) Effects of cobalt precursor on pyrolyzed carbon-supported cobalt-polypyrrole as electrocatalyst toward oxygen reduction reaction. Nanoscale Res Lett 8:478
Morales-Guio CG, Stern LA, Hu XL (2014) Nanostructured hydrotreating catalysts for electrochemical hydrogen evolution. Chem Soc Rev 43:6555–6569
Fang YH, Liu ZP (2009) Surface phase diagram and oxygen coupling kinetics on flat and stepped Pt surfaces under electrochemical potentials. J Phys Chem C 113:9765–9772
Lu YZ, Jiang YY, Chen W (2013) PtPd porous nanorods with enhanced electrocatalytic activity and durability for oxygen reduction reaction. Nano Energy 2:836–844
Gressley J, Jarmaillo TF, Bonde J, Chorkendorff I, Nørskov JK (2006) Computational high-throughput screening of electrocatalytic materials for hydrogen evolution. Nat Mater 5:909–913
Pan H, Feng YP, Lin JY (2010) Enhancement of hydrogen evolution on tungsten doped platinum. J Comput Theor Nanosci 7:547–551
Fang YH, Wei GF, Liu ZP (2013) Catalytic role of minority species and minority sites for electrochemical hydrogen evolution on metals: surface charging, coverage, and Tafel kinetics. J Phys Chem C 117:7669–7680
Yung TY, Liu TY, Huang LY, Wang KS, Tzou HM, Chen PT, Chao CY, Liu LK (2015) Characterization of Au and bimetallic PtAu nanoparticles on PDDA-graphene sheets as electrocatalysts for formic acid oxidation. Nanoscale Res Lett 10:365
Xia BY, Wu HB, Li N, Yan Y, Lou XW, Wang X (2015) One-pot synthesis of Pt-Co alloy nanowire assemblies with tunable composition and enhanced electrocatalytic properties. Angew Chem Int Ed 54:3797–3801
Kibsgaard J, Chen Z, Reinecke BN, Jaramillo TF (2012) Engineering the surface structure of MoS2 to preferentially expose active edge sites for electrocatalysis. Nat Mater 11:963–969
Qu Y, Pan H, Kwok CT, Wang ZS (2015) A first-principles study on hydrogen evolution reaction of VS2 nanoribbons. Phys Chem Chem Phys 17:24820–24825
Yang J, Shin HS (2014) Recent advances in layered transition metal dichalcogenides for hydrogen evolution reaction. J Mater Chem A 2:5979–5985
Qu Y, Pan H, Kwok CT, Wang ZS (2015) Effect of doping on hydrogen evolution reaction of vanadium disulfide monolayer. Nanoscale Res Lett 10:480
Saadi FH, Carim AI, Velazquez JM, Baricuatro JH, McCrory CCL, Soriaga MP, Lewis NS (2014) Operando synthesis of macroporous molybdenum diselenide films for electrocatalysis of the hydrogen-evolution reaction. ACS Catal 4:2866–2873
Xu K, Wang FM, Wang ZX, Zhan XY, Wang QS, Cheng ZZ, Safdar M, He J (2014) Component-controllable WS2(1–x)Se2x nanotubes for efficient hydrogen evolution reaction. ACS Nano 8:8468–8476
Chen TY, Chang YH, Hsu CL, Wei KH, Chiang CY, Li LJ (2013) Comparative study on MoS2 and WS2 for electrocatalytic water splitting. Int J Hydro Energy 38:12302–12309
Lukowski MA, Daniel AS, English CR, Meng F, Forticaux A, Hamers RJ, Jin S (2014) Highly active hydrogen evolution catalysis from metallic WS2 nanosheets. Energy Environ Sci 7:2608–2613
Lin J, Peng ZW, Wang G, Zakhidov D, Larios E, Yacaman MJ, Tour JM (2014) Enhanced electrocatalysis for hydrogen evolution reactions from WS2 nanoribbons. Adv Fun Mater 4:1301875
Pan H (2014) Metal dichalcogenides monolayers: novel catalysts for electrochemical hydrogen production. Sci Rep 4:5348
Voiry D, Salehi M, Silva R, Fujita T, Chen MW, Asefa T, Shenoy VB, Eda G, Chhowalla M (2014) Conducting MoS2 nanosheets as catalysts for hydrogen evolution reaction. Nano Lett 13:6222–6227
Lukowski MA, Daniel AS, Meng F, Forticaux A, Li LS, Jin S (2013) Enhanced hydrogen evolution catalysis from chemically exfoliated metallic MoS2 nanosheets. J Am Chem Soc 135:10274–10277
Shi JP, Ma DL, Han GF, Zhang Y, Ji QQ, Gao T, Sun JY, Song XJ, Li C, Zhang YS, Lang XY, Zhang YF, Liu ZF (2014) Controllable growth and transfer of monolayer MoS2 on Au foils and its potential application in hydrogen evolution reaction. ACS Nano 8:10196–10204
Geng XM, Wu W, Li N, Sun WW, Armstrong J, Al-hilo A, Brozak M, Cui JB, Chen TP (2014) Three-dimensional structures of MoS2 nanosheets with ultrahigh hydrogen evolution reaction in water reduction. Adv Fun Mater 24:6123–6129
Zhang XW, Meng F, Christianson JR, Arroyo-Torres C, Lukowski MA, Liang D, Schmidt JR, Jin S (2014) Vertical heterostructures of layered metal chalcogenides by van der Waals epitaxy. Nano Lett 14:3047–3052
Voiry D, Yamaguchi H, Li JW, Silva R, Alves DCB, Fujita T, Chen MW, Asefa T, Shenoy VB, Eda G, Chhowalla M (2013) Enhanced catalytic activity in strained chemically exfoliated WS2 nanosheets for hydrogen evolution. Nat Mater 13:850–855
Yu YF, Huang SY, Li YP, Steinmann SN, Yang WT, Cao LY (2014) Layer-dependent electrocatalysis of MoS2 for hydrogen evolution. Nano Lett 14:553–558
Yan Y, Xia BY, Xu ZC, Wang X (2014) Recent development of molybdenum sulfides as advanced electrocatalysts for hydrogen evolution reaction. ACS Catal 4:1693–1705
Tsai C, Chan K, Nørskov JK, Abild-Pedersen F (2015) Rational design of MoS2 catalysts: tuning the structure and activity via transition metal doping. Catal Sci Technol 5:246–253
Tsai C, Abild-Pedersen F, Nørskov JK (2014) Tuning the MoS2 edge-site activity for hydrogen evolution via support interactions. Nano Lett 14:1381–1387
Lee JH, Jang WS, Han SW, Baik HK (2014) Efficient hydrogen evolution by mechanically strained MoS2 nanosheets. Langmuir 30:9866–9873
Hohenberg P, Kohn W (1964) Inhomogeneous electron gas. Phys Rev 136:B864–B871
Blöchl PE (1994) Projector augmented-wave method. Phys Rev B 50:17953–17979
Kresse G, Furthmuller J (1996) Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys Rev B 54:11169–11186
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868
Kresse G, Joubert D (1999) From ultrasoft pseudopotentials to the projector augmented-wave method. Phys Rev B 59:1758–1775
Monkhorst HJ, Pack J (1976) Special points for brillouin-zone integrations. Phys Rev B 13:5188–5192
Pan H (2014) Electronic and magnetic properties of vanadium dichalcogenides monolayers tuned by hydrogenation. J Phys Chem C 118:13248–13253
Li H, Tsai C, Koh AL, Cai L, Contryman AW, Fragapane AH, Zhao JH, Han HS, Manoharan HC, Abild-Pedersen F, Nørskov JK, Zheng XL (2016) Activating and optimizing MoS2 basal planes for hydrogen evolution through the formation of strained sulphur vacancies. Nat Mater 15:48–53
Acknowledgements
Hui Pan thanks the support of the Science and Technology Development Fund from Macau SAR (FDCT-068/2014/A2, FDCT-132/2014/A3, and FDCT-110/2014/SB) and the Multi-Year Research Grant (MYRG2014-00159-FST and MYRG2015-00017-FST) from the Research and Development Office at the University of Macau. The DFT calculations were performed at the High Performance Computing Cluster (HPCC) of Information and Communication Technology Office (ICTO) at the University of Macau.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The author declares that he has no competing interests.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Pan, H. Tension-Enhanced Hydrogen Evolution Reaction on Vanadium Disulfide Monolayer. Nanoscale Res Lett 11, 113 (2016). https://doi.org/10.1186/s11671-016-1329-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11671-016-1329-5