Abstract
The mechanism of flat band voltage (VFB) shift for alternate La2O3/Al2O3 multilayer stack structures in different annealing condition is investigated. The samples were prepared for alternate multilayer structures, which were annealed in different conditions. The capacitance-voltage (C-V) measuring results indicate that the VFB of samples shift negatively for thinner bottom Al2O3 layer, increasing annealing temperature or longer annealing duration. Simultaneously, the diffusion of high-k material to interfaces in different multilayer structures and annealing conditions is observed by X-ray photoelectron spectroscopy (XPS). Based on the dipole theory, a correlation between the diffusion effect of La towards bottom Al2O3/Si interface and VFB shift is found. Without changing the dielectric constant k of films, VFB shift can be manipulated by controlling the single-layer cycles and annealing conditions of alternate high-k multilayer stack.
Similar content being viewed by others
Background
High dielectric constant (high-k) materials have been extensively used to substitute conventional SiO2 gate oxides for its prominent properties such as small equivalent oxide thickness (EOT) and low leakage current. During the past years, researchers have paid lots of attentions to high-k materials, such as hafnium oxide (HfO2), yttrium oxide (Y2O3), zirconium oxides (ZrO2), lanthanum oxide (La2O3), aluminum oxide (Al2O3), and other transition-metal oxides. Among them, La2O3 is considered a remarkable candidate because of its high dielectric constant (approximately 25) and large band gap (approximately 5.8 eV). However, the application of high-k materials also cause lots of new problems and challenges [1, 2]. Recently, the properties of La2O3 and Al2O3 gate stacks have been studied by many researchers, and much promotion has been made in restraining leakage current and suppressing the formation of parasitic interface [3–5].
Furthermore, flat band voltage has been regarded as one of the most critical parameters for the design and fabrication of semiconductor devices. Earlier researchers claimed that the fixed charges are the main factor for flat band voltage (VFB) shift [6]. However, Dr. Wang pointed out there was no correlation between VFB and fixed charges because the film Hf x La1 − x O y keep the same VFB for different film thicknesses [7, 8]. Researchers also revealed that the main origin of VFB is the dipoles between high-k/interface layer [9, 10]. Besides, the atomic mechanism of VFB shifts for different high-k gate stacks is also discussed specifically by Lin and Robertson [11, 12]. However, the influence of the film structure and annealing conditions on VFB has not been fully investigated. In this study, firstly, a model of VFB including the interfaces of metal/high-k and high-k/Si was introduced. Then, alternate La2O3/Al2O3 multilayer stacks were prepared with different single-layer cycles by atomic layer deposition (ALD) and annealed in different temperatures and duration. The electrical and physical characteristics of the samples were investigated. Based on the theory of dipoles and diffusion effect, the mechanism of VFB shift was studied.
Methods
Firstly, p-type Si(100) wafers were washed in deionized water and chemically etched with diluted HF for 3 min to remove the native oxide. Then, alternate La2O3/Al2O3 multilayer high-k stacks with different single-layer cycles were deposited on Si wafers by ALD reactor (Picosun R-150, Espoo, Finland) in 300 °C. La(i-PrCp)3 and trinethyluminium (TMA) were used as precursors of La and Al, respectively. Besides, O3 was used as oxidant, and ultra-high purity nitrogen (N2, 99.999 %) was employed as carrier and purge gas. After deposition, the rapid thermal annealing (RTA) process was carried out at different temperatures for different duration in N2 ambient. For further analysis, annealed La2O3/Al2O3 film thickness (without metal gate) was examined by Woollam M2000D (Woollam Co. Inc., Lincoln, NE, USA) spectroscopic ellipsometry (SE). X-ray photoelectron spectroscopy (XPS) was used to examine the bonding structures and chemical quantitative composition of the films. C1s peak from adventitious carbon at 284.6 eV [13] was used as an internal energy reference during the analysis. Besides, 100-nm-thick Al was deposited by magnetron sputtering as electrode, and then, capacitance-voltage (C-V) measurement was carried out using Agilent B1500A semiconductor analyzer at the frequency of 100 kHz.
Results and Discussion
Taking into consideration of fixed charges and interfacial dipoles, the VFB of conventional metal/SiO2/Si metal oxide semiconductor (MOS) structure can be expressed as [14]:
where φ ms is the work function difference between metal and Si substrate and Q 0 represents the fixed charges located in oxide layer. Δmetal/SiO2 and ΔSiO2/Si are dipoles located in the interface of metal/SiO2 and SiO2/Si.
In this work, SiO2 is substituted by high-k materials Al2O3 and La2O3, so the VFB of samples with alternate high-k dielectric gate stacks can be expressed as:
In this equation, Q 0 and Q 1 represent the fixed charges in Al2O3 and La2O3 films, respectively. As shown in Fig. 1, all dipoles can be separated into three kinds. They are the dipoles between the alternate high-k films, the dipoles at interfaces of metal/La2O3, and dipoles at interfaces of Al2O3/Si. Between the alternate high-k layers, the dipoles La–O–Al and Al–O–La have reverse sequence which can cancel out each other. So these dipoles do not create net dipole.
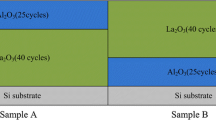
Furthermore, some researches have proved that the contribution of dipoles at metal/high-k interface to the VFB shift can be neglected [10, 15, 16]. For inspecting this point of view, two samples were prepared with different high-k films, and then, the RTA process was carried out at 600 °C for 60 s in N2 atmosphere. Their simplified schematic structures and C-V curves are shown in Fig. 2. It should be noted that the two films show approximately the same VFB: 1.49 V (without La2O3 inserted layer) and 1.47 V (with La2O3 inserted layer). The insensitiveness of VFB values to the kind of dipoles at metal/high-k interface clearly indicates the metal/high-k interface is not one of the origin of VFB shift. Therefore, in this work, the fixed charges Q 0 and Q 1 and the dipoles at interface of Al2O3/Si need to be examined on the next step.
Samples S1~S5 were deposited for different structures in an identical annealing condition (annealed at 600 °C for 60 s in N2 atmosphere). The schematic of alternate high-k films S1~S5 is shown in Fig. 3. For each of the samples S1~S4, 40-cycle La2O3 and 40-cycle Al2O3 were deposited with different single-layer cycles (from S1 to S4 are 40, 20, 10, and 1 cycle of single layer). In order to investigate the influence of fixed charges on VFB, as shown in Fig. 3b, e, the sample S5 was deposited with the same single-layer cycles but double number of layers as S2. The structures and thicknesses of samples S1~S5 are listed in Table 1.
Figures 4 and 5 show the C-V curves and VFB shifts of samples S1~S5. Samples S1~S4 have very close accumulation capacitance. The EOTs of samples S1~S5 are extracted by NCSU CVC program [17], which are 2.21, 2.20, 2.21, 2.29, and 4.16 nm, respectively. The dielectric constants are 12.46, 12.44, 12.34, 12.89, and 12.56, respectively. The VFB of samples S1~S5 are 1.45, 1.30, 0.60, 0.25, and 1.30 V, respectively. For 80-cycle pure Al2O3 and 80-cycle pure La2O3 films deposited and annealed in the same condition with S1~S5, VFB are 1.49 and −0.32 V, which is shown in Fig. 5. We notice that VFB become smaller shifting from the VFB of pure Al2O3 film to the pure La2O3 direction for a thinner single layer (from 40 to 1 cycle). In a recent report [10], a negative VFB shift is observed for a thicker La2O3 inserted layer at HfO2/Si interface which comes to a similar conclusion with our work. Furthermore, VFB is the same for samples S2 and S5, which indicates the Al2O3 and La2O3 films have few fixed charges. Therefore, the fixed charge Q 0 and Q 1 in Eq. 2 can be neglected for studying VFB shift. As discussed, the VFB shifts of S1~S4 have no relevance to the dipoles between alternate high-k layers and dipoles at metal/high-k interface. Therefore, it is clear that such a shift (from 1.45 to 0.25 V) is supposed to be relevant to the variation of dipoles at Al2O3/Si interface.
For further investigation about the mechanism of VFB shift for high-k gate stacks, different annealing conditions were employed after the ALD deposition. As shown in Table 2, samples S2 and S6~S10 were deposited for identical structure and then annealed at different temperatures (600, 700, and 800 °C) for different duration (30, 60, 90, and 120 s) in N2 atmosphere. The correlations between C-V curves and annealing conditions of samples S2 and S6~S10 are shown in Fig. 6. The EOTs of samples S2 and S6~S10 extracted by NCSU CVC program [17] are 2.20, 2.23, 2.29, 2.20, 2.20, and 2.20 nm, respectively, and the dielectric constant k can be figured out as 12.44, 12.61, 12.26, 12.55, 12.53, and 12.62, respectively. As shown in Fig. 7, the VFB of samples S2 and S6~S10 are 1.3, 0.75, 0.5, 1.33, 1.28, and 1.22 V, respectively, which have a remarkable negative shift with increasing annealing temperature and a slight negative shift with increasing duration. Similar trend of VFB shift (approximately 1 to 0.6 V) was also reported for HfO2 and Al2O3 stacks at different annealing temperature (400 and 1000 °C, respectively) [9].
Then, XPS was employed to examine the variation of bonding structure. Figure 8 shows the O1s XPS spectra of annealed samples S1~S4, and each of the spectra was fitted with four peaks Si–O–Al (532.5 eV), Al–O–Al (531.5 eV), Al–O–La (530.9 eV), and La–O–La (528.75 eV). It is found that La–O–Al peaks become larger while Al–O–Al and La–O–La peaks become smaller from S1 to S4. That is attributed to more La2O3/Al2O3 interface layers formed with decreasing single-layer cycles. As we discussed above, the dipoles La–O–Al and Al–O–La can cancel each other, so the variation of these peaks makes no contribution to VFB shift.
Figure 9 show the O1s XPS spectra of samples S2, S6, and S7. More Al–O–La bonds and less Al–O–Al and La–O–La bonds are observed from S2 to S7, which indicates more Al2O3 and La2O3 diffuse into each other and form LaAlO3 at Al2O3/La2O3 interface at higher annealing temperature. The value of diffusion coefficient mainly depends on the kinds of diffusion substance and diffusion medium as well as the diffusion temperature. So this trend is due to the larger diffusion coefficient obtained at higher temperature.
In both Figs. 8 and 9, we notice that there is only small amount of Si–O–Al bonds which show a slight decrease for thinner single layer or higher annealing temperature. Thinner single layer means a thinner bottom Al2O3 layer, which leads to more La2O3 diffusing into Al2O3/Si interface and replacing the Si–O–Al with Si–O–La bonds. Based on the theory of diffusion, more La2O3 can diffuse through the bottom Al2O3 layer forming Si–O–La bonds at higher annealing temperature. Similarly, increasing annealing duration can also cause more La2O3 diffusing into Al2O3/Si interface. That is why, the amount of Si–O–Al bonds declines. This diffusion effect of high-k material after annealing process is also proved in report [9], which shows HfO2 and Al2O3 stacks diffusing into each other and into the metal/high-k and high-k/Si interfaces after annealing process at different temperatures.
On the other hand, Si–O–Al and Si–O–La bonds are located at the interface of high-k/Si. According to the dipole theory discussed above, this substitution of La for Al should be responsible for the negative VFB shift. Therefore, we should also discuss how the increasing La–O–Si bonds at Al2O3/Si interface influences the VFB. La has weaker electronegativity than Al (La ~ 1.11, Al ~ 1.61). When Al is substituted by La, compared with Al, electrons will be further from La and move towards O. So the dipole La–O presents a larger polarity compared with dipole Al–O. It means a larger electrostatic potential, which can increase the band offset and finally cause the VFB shift. It is concluded that the more Al is substituted by La at Al2O3/Si interface the closer to blue dotted line, the VFB will be. Moreover, the VFB shift in different annealing temperatures is much bigger compared with different duration. It can be explained by the exponential relationship between the temperature and diffusion coefficient. Therefore, a feasible way to modulate the VFB of alternate high-k multilayer stack gate is to control the single-layer cycles and annealing conditions.
In addition, we should notice that the diffusion should be bidirectional, meaning the Al2O3 should also diffuse into the metal/La2O3 interface. But the experiment in our work shows no relevance between the metal/high-k interface and the VFB shift. In fact, some researchers hold the contrary opinions to our work by investigating the high-k inserted layer at metal/high-k interface. However, in these reports [18, 19], the high temperature annealing (1000 °C) and thin high-k films (approximately 4 nm) may lead to the non-negligible diffusion of inserted layer material to the high-k/Si interface and result in VFB shift. Furthermore, the reason that dipoles at metal/high-k and high-k/Si interface present distinct effect on VFB shift is supposed to be relevant to the different properties of bonds. Unlike La–O–Si or Al–O–Si, the bonds between metal gate and high-k material are ionic bonding (La–O–Al or Al–O–Al), which may result in distinctly different electrical properties of dipoles. However, further work is still underway towards a more specific explanation about that.
Conclusions
The C-V curves and XPS results of alternate La2O3/Al2O3 multilayer stacks are investigated in the paper. It is concluded that the main factor of VFB shift is the dipoles at high-k/Si interface. Furthermore, the VFB of samples shifts negatively and varies from the VFB of pure Al2O3 to the pure La2O3 direction for thinner bottom Al2O3 layer, increasing annealing temperature or longer annealing duration. In such a condition, more La2O3 can diffuse into the Al2O3/Si interface and form La–O–Si bonds. Because of a weaker electronegativity of La, dipole La–O has stronger polarity than dipole Al–O. It leads to the band offset and negative VFB shift. As a result, a feasible way to modulate VFB without changing the dielectric constant k of films is proposed.
Abbreviations
- ALD:
-
Atomic layer deposition
- C-V:
-
Capacitance-voltage
- EOT:
-
Equivalent gate oxide thickness
- MOS:
-
Metal oxide semiconductor
- RTA:
-
Rapid thermal annealing
- SE:
-
Spectroscopic ellipsometry
- TMA:
-
Trinethyluminium
- VFB :
-
Flat band voltage
- XPS:
-
X-ray photoelectron spectroscopy
References
Zhao Y, Kita K, Kyuno K et al (2009) Band gap enhancement and electrical properties of La2O3 films doped with Y2O3 as high-k gate insulators. Appl Phys Lett 94:042901
Cao D, Cheng X, Yu Y et al (2013) Competitive Si and La effect in HfO2 phase stabilization in multi-layer (La2O3)0.08(HfO2) films. Appl Phys Lett 103:081607
Wang X, Liu HX, Fei CX et al (2015) Silicon diffusion control in atomic-layer-deposited Al2O3/La2O3/Al2O3 gate stacks using an Al2O3 barrier layer. Nanoscale Res Lett 10:1–6
Lee WJ, Ma JW, Bae JM et al (2013) The diffusion of silicon atoms in stack structures of La2O3 and Al2O3. Curr Appl Phys 13:633–639
Kim Y, Woo S, Kim H et al (2010) Effects of an Al2O3 capping layer on La2O3deposited by remote plasma atomic layer deposition. J Mater Res 25:1898–1903
Lee JH, Koh K, Lee NI et al (2000) Effect of polysilicon gate on the flatband voltage shift and mobility degradation for ALD-Al2O3 gate dielectric. In: International Electron Devices Meeting., pp 645–648
Wang XP, Li MF, Ren C et al (2006) Tuning effective metal gate work function by a novel gate dielectric HfLaO for nMOSFETs. IEEE Electron Device Lett 27:31–33
Wang XP, Lim EJ, Yu HY et al (2007) Work function tunability of refractory metal nitrides by lanthanum or aluminum doping for advanced CMOS devices. IEEE Transact Electron Devices 54:2871–2877
Kornblum L, Meyler B, Cytermann C et al (2012) Investigation of the band offsets caused by thin Al2O3 layers in HfO2 based Si metal oxide semiconductor devices. Appl Phys Lett 100:062907
Kakushima K, Okamoto K, Adachi M et al (2008) Origin of flat band voltage shift in HfO2 gate dielectric with La2O3 insertion. Solid-State Electr 52:1280–1284
Lin L, Robertson J (2009) Atomic mechanism of flat-band voltage shifts by La2O3 and Al2O3 in gate stacks. Appl Phys Lett 95:012906
Lin L, Robertson J (2009) Atomic mechanism of flat-band voltage shifts at La2O3, Al2O3 and Nb2O5 capping layers. Microelectr Eng 86:1743–1746
Pelloquin S, Saint-Girons G, Baboux N et al (2013) LaAlO3/Si capacitors: comparison of different molecular beam deposition conditions and their impact on electrical properties. J Appl Phys 113:034106
Kaushik VS, O'Sullivan BJ, Pourtois G et al (2006) Estimation of fixed charge densities in hafnium-silicate gate dielectrics. IEEE Transact Electron Devices 53:2627–2633
Yamamoto Y, Kita K, Kyuno K et al (2007) Study of La-induced flat band voltage shift in metal/HfLaOx/SiO2/Si capacitors. Japanese J Appl Phys 46:7251–7255
Iwamoto K, Kamimuta Y, Ogawa A et al (2008) Experimental evidence for the flatband voltage shift of high-k metal-oxide-semiconductor devices due to the dipole formation at the high-k/SiO2 interface. Appl Phys Lett 92:132907
Hauser J (2000) NCSU CVC software, version 7.0. Raleigh, USA: Department of Electrical and Computer Engineering, North Carolina State University
Pantisano L, Schram T, Osullivan B et al (2006) Effective work function modulation by controlled dielectric monolayer deposition. Appl Phys Lett 89:113505
Narayanan V, Paruchuri V, Bojarczuk N et al (2006) Band-edge high-performance high-k/metal gate n-MOSFETs using cap layers containing group IIA and IIIB elements with gate-first processing for 45 nm and beyond. In: VLSI Technology., pp 178–179
Acknowledgements
This research is supported by the National Natural Science Foundation of China (Grant Nos. 61376099 and 61434007) and the Foundation for Fundamental Research of China (Grant No. JSZL2016110B003).
Authors’ contributions
XyF generated the research idea, analyzed the data, and wrote the paper. XyF and XW carried out the experiments and the measurements. XW, LZ, CxF, and HlL participated in the discussions. HxL has given the final approval of the version to be published. All authors read and approved the final manuscript.
Authors’ information
XyF and HlL are Master students in the Xidian University. HxL is a professor in the Xidian University. XW, LZ, and CxF are PhD students in the Xidian University.
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Feng, XY., Liu, HX., Wang, X. et al. Impacts of Annealing Conditions on the Flat Band Voltage of Alternate La2O3/Al2O3 Multilayer Stack Structures. Nanoscale Res Lett 11, 394 (2016). https://doi.org/10.1186/s11671-016-1623-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11671-016-1623-2