Abstract
Approximately single-domain-sized 9-, 13-, and 16-nm CoFe2O4 nanoparticles are synthesized using the thermal decomposition of a metal-organic salt. By means of dilution and reduction, the concentration, moment, and anisotropy of nanoparticles are changed and their influence on the magnetic properties is investigated. The relation of M r/M s ∝ 1/lgH dip is observed, where M r/M s is the remanence ratio and H dip is the maximum dipolar field. Especially, such relation is more accurate for the nanoparticle systems with higher concentration and higher moment, i.e., larger H dip. The deviation from M r/M s ∝ 1/lgH dip appearing at low temperatures can be attributed to the effects of surface spins for the single-phase CoFe2O4 nanoparticles and to the pinning effect of CoFe2O4 on CoFe2 for the slightly reduced nanoparticles.

Approximately single-domain-sized 9-, 13-, and 16-nm CoFe2O4 nanoparticles were synthesized and then the concentration, moment, and anisotropy of these NPs were changed. The correlation of M r/M s ∝ 1/lgH dip was observed, independent of the size, concentration, moment, and anisotropy, and especially, such correlation is more accurate for the nanoparticle systems with higher concentration or moment, i.e., stronger dipolar interaction, which has not been reported before as far as we know.
Similar content being viewed by others
Background
Nanoscale magnetic materials often exhibit novel properties, differing from those of their bulk polycrystalline counterparts [1–5], as a result of several effects including the finite size effect, surface effect, and interparticle interaction [6–8]. These effects affect the magnetic properties and the magnetic ordering state of nanoparticles (NPs) individually, and sometimes synergetically which usually occurs in the dense magnetic NPs. One typical phenomenon of the size effect is that the coercivity (H c) reaches the maximum as the particle size (D) decreases to a single-domain critical dimension D c, and then reduces monotonically to zero when D is further decreased to a certain size below D c [9]. Concomitantly, the NPs exhibit the superparamagnetic behavior with the theoretical remanence (M r) to saturation (M s) magnetization ratio (M r/M s) being zero. Surface spin is another factor to affect the magnetic properties for the nano-sized magnetic materials. The total magnetization of a nanoparticle composes of the surface and core spins [10, 11], which is known as the core-shell magnetization model. Surface spins reduce the magnetization of a magnetic nanoparticle due to the disorder of spins at the nanoparticle surface, and the disordered surface spins lower the critical magnetic ordering temperature of magnetic nanoparticles compared with that of the bulk material [12]. Below a certain temperature, the canted surface spins freeze into a spin-glass-like state and the hysteresis loops obtained after a field cooling (FC) shift, as a consequence of the unidirectional anisotropy resulting from the coupling between the disordered surface layer and core spins [6, 13, 14]. Furthermore, the interaction between the surface spins of different particles enhances the effective anisotropy [7, 15], making the surface anisotropy constant many orders higher than that of the bulk material [16].
Apart from surface spins, the interparticle dipolar interaction (IPDI) widely exists in the magnetic NPs; it plays a complex role in magnetic properties. For example, it has been suggested that the IPDI enhances H c because of additional induced anisotropy [17], while the opposite conclusion has also been observed [18]. A strong IPDI decreases the M r/M s ratio, which has been proved both theoretically and experimentally [18–23]. Furthermore, it is well known that the strength of IPDI depends on the concentration of magnetic NPs and obviously affects the magnetic ordering states: in a heavily diluted system with low concentration of magnetic NPs, the system exhibits superparamagnetism [24], while in a dense system with high concentration, the NPs exhibit the super-spin glass (SSG) states at low temperature [25, 26].
So far, the effects of IPDI on the magnetic properties have not been comprehensively investigated, instead they have been reported piecemeal by different researchers. The strength of IPDI, which can be expressed by the maximum dipolar field H dip, defined as H dip = 2 μ/d 3, where μ is the particle moment (μ = M s × V m; M s is saturation magnetization and V m is magnetic grain volume) and d is the distance between particles (center to center), which is inversely proportional to the NP concentration. The purpose of the present work is to systematically reveal the effects of H dip on the M r/M s ratio for the 9-, 13-, and 16-nm magnetic NPs with different concentrations, particle moments, and anisotropies.
Methods
Experimental Procedure
Preparation of CoFe2O4 NPs
Co(acac)2 (97 %; acac is acetylacetonate), Fe(acac)3 (98 %), benzyl ether (97 %), oleic acid (90 %), and oleylamine (80–90 %) were mixed in a 1000-ml three-necked round-bottom flask by magnetic stirring under a flow of nitrogen (99.999 %). The mixture was heated at 120 °C for 0.5 h to remove air and moisture, at 200 °C under reflux for 2 h, and then at 290 °C for 1 h. After the mixture was cooled naturally to room temperature, absolute ethanol was added to produce a precipitate. The precipitate was separated via centrifugation and then washed with absolute ethanol several times to obtain CoFe2O4 NPs. By varying the intermediately treating temperature, we prepared 9-, 13-, and 16-nm CoFe2O4 NPs.
Dilution of CoFe2O4 NPs in a SiO2 Matrix
In order to change the interparticle distance, some of the 9- and 13-nm CoFe2O4 NPs were diluted in a SiO2 matrix with different concentrations, because the interparticle distance is inversely proportional to the concentration. CoFe2O4 NPs were added to a solution of cyclohexane (400 ml), polyethylene glycol (25 ml), tetraethyl orthosilicate, and ammonia, and then stirred mechanically for 24 h. Ethanol was added to form a precipitate. The precipitate was isolated by centrifugation and then washed with ethanol and water to remove unreacted molecules. The precipitate was dried at 80 °C for 6 h to obtain the diluted CoFe2O4 NPs by SiO2. By increasing the relative content of CoFe2O4, we prepared the CoFe2O4 NPs with low, moderate, and high concentrations, and the samples are referred to as L9, M9, and H9 for 9-nm NPs and as L13, M13, and H13 for 13-nm NPs.
Reduction of CoFe2O4 NPs
In order to change the moment and anisotropy of NPs, the reduction reactions were performed in the H2/N2 atmosphere (500 sccm, 96 % N2 + 4 % H2) to prepare a composite of CoFe2O4 and CoFe2 alloy, because the CoFe2 alloy is a typical soft ferromagnet with high moment and small anisotropy, compared with CoFe2O4. To avoid the aggregation of NPs during reduction, the 16-nm NPs were first diluted or separated by SiO2 and then reduced at low, moderate, and high temperatures at 300, 400, and 500 °C for 4 h, and the obtained samples are denoted as LT16, MT16, and HT16.
Characterization
The crystal structure of the products was determined by X-ray diffraction (XRD) using an X-ray diffractometer (DX-2000 SSC) with Cu Kα irradiation (λ = 1.5406 Å) in the scanning range 20°–80° with a step size of 0.02°. (High-resolution) transmission electron microscopy ((HR)TEM; JEOL, JEM-2100) was used to observe the morphology characteristics. Magnetic measurements were carried out using a superconducting quantum interference device PPMS system (Quantum Design, PPMS EC-II).
Results and Discussion
Crystal Structure and Morphology
TEM images and size histograms in Fig. 1 show that the NPs are ca. 9, 13, and 16 nm. Most of 9- and 13-nm NPs exhibit the spherical-like morphology, while some 16-nm NPs exhibit rhombohedral and quadrate shapes. The size distribution range is ca. 2 nm for the 9-nm NPs and 4 nm for 13- and 16-nm NPs.
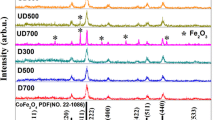
The XRD experiments have been performed on all samples, and herein, we show the results of several samples representatively. As shown in Fig. 2b–d, the as-prepared and undiluted 9-nm (the H9 sample), 13-nm (the H13 sample), and 16-nm CoFe2O4 NPs are single phases and have the cubic spinel structure, according to the standard powder diffraction file (PDF) of CoFe2O4 (No. 22-1086) in Fig. 2a. For the diluted 16-nm NPs after reduction at 400 °C, i.e., the MT16 sample, the extra diffraction peaks, besides those from CoFe2O4, can be assigned to the reflection from (110) and (200) crystallographic planes of the CoFe2 alloy, according to the PDF card of CoFe2 (No. 65-4131) in Fig. 2f. This XRD result indicates that CoFe2O4 is partially reduced to CoFe2 due to the reaction: CoFe2O4 + 4H2 → CoFe2 + 4H2O [27]. To observe the existence of CoFe2 in the reduced sample, the (HR)TEM was ever performed on the undiluted sample. The lattice fringes of CoFe2 can be observed at the surface of particle, i.e., CoFe2 exists at the outer layer while CoFe2O4 exists in the inner of particle.
Magnetic Properties
The dependence of the magnetization (M) of all samples on the applied magnetic field (H), i.e., M(H) loop (−70 kOe < H < 70 kOe) was measured at temperatures of 10, 50, 100, 150, 200, 250, 300, and 390 K. Representatively, the M(H) loops recorded at 10 and 390 K were shown in Fig. 3 for 9- and 13-nm NPs with different concentrations and in Fig. 4 for diluted 16-nm NPs with different reducing temperatures. From these loops, the coercivity (H c), saturation magnetization (M s), and remanence (M r) to saturation magnetization ratio (M r/M s) values at different temperatures can be obtained. In the case of the CoFe2O4 NPs with moderate (Fig. 3b, e) and high (Fig. 3c, f) concentrations, the loops recorded at 10 K show a jump around H = 0; this phenomenon is common for magnetic NPs and can be assigned to the reorientation of surface spins around NPs [28, 29]. However, in the case of the CoFe2O4 NPs with low concentration, as shown in Fig. 3a, d, the 10-K loops become smooth which is characteristic of a single-phase hard magnet, and the possible reason is that the surface spins are strongly pinned by the SiO2 matrix.
For the diluted and reduced 16-nm NPs, containing CoFe2O4 and CoFe2 phases, as shown in Fig. 4, the jump around H = 0 becomes higher as the reducing temperature increases, as a result of the increase in the relative CoFe2 content [30, 31]. Herein, the jump is attributable to the different reversal fields of hard CoFe2O4 and soft CoFe2, which is an indicative of no exchange-coupling occurring between soft and hard species, because in an exchange-coupled system, the magnetization could show an equivalent reversal behavior over the whole temperature region and the loop should be as smooth as that of the single-phase hard magnet. With increasing temperature, the anisotropy field of CoFe2O4 decreases markedly, so the average reversal fields of hard and soft phases may be similar, resulting in the single-phase behavior of the loops (not shown here).
The undiluted 9- and 13-nm NPs, i.e., H9 and H13, as well as the 16-nm NPs reduced at 300 °C (LT16) have the H c values of 20.0, 18.8, and 20.5 kOe at 10 K, smaller than 23.8 kOe (10 K) for our previously reported 10.7-nm CoFe2O4 NPs [32] which particle size is close to the critical size of a single domain [33]. These H c values are much larger than that for the bulk CoFe2O4 (6.8 kOe at 10 K) [34], because on the one hand, the particles are approximately single-domain sized, and on the other hand, the interaction between surface spins induces extra anisotropy and hence enhances H c, which will be further discussed below. As the temperature increases, H c monotonically decreases. The decrease in coercivity can be attributable to thermal fluctuations of the blocked moment, across the anisotropy barrier. For an assembly of non-interacting single-domain magnetic nanoparticles with uniaxial anisotropy, the coercivity can be written in the form of simple model of thermal activation of particle moments over the anisotropy barriers (Kneller’s law) as [6, 35] H c = H c0[1 − (T/T B)1/2], where H c0 is the value of H c at 0 K and T B denotes the blocking temperature. The H c values of all samples can be fitted to Kneller’s law in the temperature range of 10–390 K. Representatively, the experimental (solid circles) and fitting curves (solid lines) of the samples H9 (a), H13(b), and LT300 (c) are plotted in Fig. 5. The obtained fitting parameter T B will be used to calculate the volume of magnetic grain, V m, as discussed below.
In the case of non-interacting and randomly oriented spherical particles with cubic anisotropy, H c obeys the relation of H c = 0.64 K/M s [15], where K is the anisotropy constant, so the K value can be calculated for all samples. The highest K for CoFe2O4 NPs at 10 K reaches ~107 erg/cm3, much larger than 1.8–3.0 × 106 erg/cm3 for the bulk CoFe2O4; this is consistent with the previous reports that in thin films and nanoparticles, surface anisotropy constant is found higher by many orders of magnitude than that of the bulk [15, 16]. In narue the enhanced K value results from the interaction between the surface spins of different particles [7], and between the spins from the surface and core in a single NP [6, 13]. Large K value induced by the surface spins leads to the increase of H c due to H c ∝ K. It should be mentioned that the fitting according to Kneller’s law and the calculation of K from H c = 0.64 K/M s are based on an assumption that nanoparticles do not interact with each other. The real situation is that the dipolar interaction widely exists in the system of magnetic nanoparticles; however, the dipolar interaction is weak, compared with the anisotropy. Therefore, such the assumption has usually been considered to be reasonable in many previous reports [6, 15, 35].
Given T B and K, the magnetic grain sizes (V m) of the nanoparticles can be estimated according to Stoner-Wohlfarth expression [36]: 25k B T B = KV m, where k B is the Boltzmann constant. Subsequently, the diameter D m of a magnetic grain for all samples can be obtained, as shown in Fig. 6.
The D m values of the 9- and 13-nm NPs are smaller than D TEM below about 250 K, because of the canted surface spin layer surrounding magnetic particles [37]. Above this temperature, D m becomes larger than D TEM because the surface spins become able to thermally fluctuate, so they can be polarized by the core moments [38]. Subsequently, the collective behavior of several particle moments promoted by the IPDI leads to an increase of D m [28]. In the case of the diluted and reduced 16-nm NPs, D m of the HT16 sample is much larger than that for the LT16 and MT16 samples as a result of the increase of strong magnetic CoFe2.
Based on the obtained V m values, the strength of IPDI can be estimated by calculating H dip according to H dip = 2 μ/d 3, where μ is the particle moment (μ = M s × V m; M s is saturation magnetization and V m is magnetic grain volume). The inverse of the logarithm of H dip, i.e., 1/lgH dip values and M r/M s ratios with added error bars (5 %) are plotted against temperature (T) in Figs. 7, 8, and 9 for 9-, 13-, and 16-nm NPs, respectively.
Next, we will discuss the correlation between M r/M s and H dip. As seen in Figs. 7 and 8, the correlation between M r/M s and 1/lgH dip roughly follows M r/M s ∝ 1/lgH dip within a reasonable error range for 9- and 13-nm NPs, even though the NPs have different size and concentration. It can be noticed from Figs. 7 and 8 that the slope of the M r/M s curve is smaller than that for the 1/lgH dip curve at low temperatures, resulting in the deviation between M r/M s and 1/lgH dip, which may result from the several competing effects, including the surface effects, finite size effects, and interparticle interactions, that are sometimes difficult to isolate in these nanoparticles, as suggested by Maaz [6]. As discussed in Fig. 6, the surface spins exist around the NP at low temperatures. Therefore, it is reasonable to suggest that the smaller slope of M r/M s may originate from the interaction between spins of surface and core and between surface spins of the neighboring particles.
In the case of the diluted and reduced 16-nm NPs which contain CoFe2O4 and CoFe2, as seen in Fig. 9, the obvious deviation between M r/M s and 1/lgH dip for the slightly reduced LT16 and MT16, while M r/M s matches well with 1/lgH dip for the heavily reduced HT16, which can be assigned to the interaction in CoFe2O4/CoFe2 NPs. The interactions in CoFe2O4/CoFe2 NPs occur at the interface (intraparticle) of CoFe2O4 and CoFe2 and from the contact between NPs (interparticle). CoFe2O4 has a large magnetic anisotropy, so that it can exert a pinning action on the CoFe2 phase, similar to the pinning effect of Fe oxide on Fe in Fe/Fe oxide NPs [38], which is the possible reason for the deviation between M r/M s and 1/lgH dip. This suggestion may be supported by the results of the irreversible magnetization reversal field (H irr) [27], defined as the magnetic field where the derivative (dM/dH) of the virgin curve has a peak. Figure 10 shows the field derivative dM/dH of the virgin curves for LT16 (a), MT16 (b), and HT16 (c) samples at 10 K. Two peaks locate at H irr = 7.90 and 22.46 kOe for the LT16 sample, at H irr = 2.28 and 22.60 kOe for the MT16 sample, and at H irr = 1.75 and 11.70 kOe for the HT16 sample. The lower field corresponds to H irr of CoFe2, because pure CoFe2 is a typical soft magnet and its H irr is about 0.9 kOe at 10 K (not shown here), while the higher field corresponds to H irr of hard CoFe2O4. The LT16 sample contains more CoFe2O4 and less CoFe2, and CoFe2O4 exerts the pinning effect on the moment of CoFe2, leading to the highest H irr of CoFe2 among three samples. With increasing the reduction temperature, the sample contains more CoFe2, and therefore the moment of CoFe2 cannot be fully pinned by CoFe2O4, leading to the lower H irr of CoFe2 for the samples MT16 and HT16. Possibly, more CoFe2 in HT16 polarizes the moments of CoFe2O4, resulting in the smaller H irr of CoFe2O4 than that of LT16 [38]. These interactions in CoFe2O4/CoFe2 NPs affect the moment reversal and consequently affect the M r/M s ratio.
However, compared with LT16 and MT16, the heavily reduced HT16 has the higher CoFe2 content as a result of reduction at a higher temperature 500 °C. Therefore, HT16 has larger particle moment, and hence stronger interparticle dipolar interaction which overcomes other effects such as surface spins and interaction between CoFe2O4 and CoFe2, consequently making the correlation between M r/M s and 1/lgH dip obey M r/M s ∝ 1/lgH dip.
Conclusions
Well-dispersed uniform CoFe2O4 NPs with sizes of 9-, 13-, and 16-nm were synthesized. Some 9- and 13-nm NPs were diluted in a SiO2 matrix to change their concentration that is inversely proportional to interparticle distance, and some diluted 16-nm NPs were reduced by H2 at 300, 400, and 500 °C to change the moment and anisotropy of the NPs. These samples were used as model systems to reveal the intrinsic correlation between M r/M s and IPDI, the strength of which was estimated by H dip.
For the diluted 9- and 13-nm NPs that were not reduced, the correlation between M r/M s and H dip follows M r/M s ∝ 1/lg H dip, regardless of the particle size and distance. Slight deviation from M r/M s ∝ 1/lg H dip, occurring at low temperatures, can be attributed to the effects of surface spins. In the case of the diluted and reduced 16-nm NPs, the relation between M r/M s and H dip deviates M r/M s ∝ 1/lgH dip for the slightly reduced NPs at 300 and 400 °C because of the pinning effect of CoFe2O4 on CoFe2. However, the heavily reduced NPs at 500 °C follows M r/M s ∝ 1/lg H dip because the strong interparticle dipolar interaction is a dominant factor to affect M r/M s.
Abbreviations
- (HR) TEM:
-
(High-resolution) transmission electron microscopy
- IPDI:
-
Interparticle dipolar interaction
- PDF:
-
Standard powder diffraction file
- SSG:
-
Super-spin glass
- XRD:
-
X-ray diffraction
References
Liang YC, Hsia HY (2013) Growth and crystallographic feature-dependent characterization of spinel zinc ferrite thin films by RF sputtering. Nanoscale Res Lett 8:537, pp8
Li XH, Xu CL, Han XH, Qiao L, Wang T, Li FS (2010) Synthesis and magnetic properties of nearly monodisperse CoFe2O4 nanoparticles through a simple hydrothermal condition. Nanoscale Res Lett 5:1039–1044
Martha SC, Martínez-Luévanos A, García-Cerda LA, Rodríguez-Fernández OS, Fuentes AF, Romero-García J, Montemayor SM (2015) Montemayor, nanostructured pure and substituted cobalt ferrites: fabrication by electrospinning and study of their magnetic properties. J Alloy Compd 653:290–297
Du DJ, Yue WB, Ren Y, Yang XJ (2014) Fabrication of graphene-encapsulated CoO/CoFe2O4 composites derived from layered double hydroxides and their application as anode materials for lithium-ion batteries. J Mater Sci 49:8031–8039
Jiang CP, Leung CW, Pong PWT (2016) Magnetic-field-assisted assembly of anisotropic superstructures by iron oxide nanoparticles and their enhanced magnetism. Nanoscale Res Lett 11:189
Mazz K, Usman M, Karim S, Mumtaz A, Hasanain SK, Bertino MFJ (2001) Magnetic response of core-shell cobalt ferrite nanoparticles at low temperature. J Appl Phys 105:13917
Blanco-Gutierrez V, Virumbrales M, Saez-Puche R, Maria J (2013) Superparamagnetic behavior of MFe2O4 nanoparticles and MFe2O4/SiO2 composites (M: Co, Ni). J Phys Chem C 117:20927–20935
Ji JY, Shih PH, Chan TS, Ma YR, Wu SY (2015) Magnetic properties of cluster glassy Ni/NiO core–shell nanoparticles: an investigation of their static and dynamic magnetization. Nanoscale Res Lett 10:243
Avazpour L, Khajeh MAZ, Toroghinejad MR, Shokrollahi H (2015) Synthesis of single-phase cobalt ferrite nanoparticles via a novel EDTA/EG precursor-based route and their magnetic properties. J Alloy Compd 637:497–503
Obaidat IM, Mohite V, Issa B, Tit N, Haik Y (2009) Predicting a major role of surface spins in the magnetic properties of ferrite nanoparticles. Cryst Res Technol 44(5):489–494
Xu ST, Ma YQ, Xu YF, Sun X, Geng BQ, Zheng GH, Dai ZX (2014) The effects of surface spin on magnetic properties of weak magnetic ZnLa0.02Fe1.98O4 nanoparticles. Nanoscale Res Lett 9:545
Jing PP, Du JL, Jin CD, Wang JB, Pan LN, Li JN, Liu QF (2016) Improved coercivity and considerable saturation magnetization of cobalt ferrite (CoFe2O4) nanoribbons synthesized by electrospinning. J Mater Sci 51:885–892
Ceylan A, Hasanain SK, Shah SI (2008) Experimental observations of field-dependent activation of core and surface spins in Ni-ferrite nanoparticles. J Phys Condens Matter 20:195208
Peddis D, Cannas C, Piccaluga G, Agostinelli E, Fiorani D (2010) Spin-glass-like freezing and enhanced magnetization in ultra-small CoFe2O4 nanoparticles. Nanotechnology 21:125705
Laureti S, Varvaro G, Testa AM, Fiorani D, Agostinelli E, Piccaluga G, Musinu A, Ardu A, Peddis D (2011) Magnetic interactions in silica coated nanoporous assemblies of CoFe2O4 nanoparticles with cubic magnetic anisotropy. Nanotechnology 21:315701
Prado Y, Mazerat S, Rivière E, Rogez G, Gloter A, Stéphan O, Catala L, Mallah T (2014) Magnetization reversal in CsNiIICrIII(CN)6 coordination nanoparticles: unravelling surface anisotropy and dipolar interaction effects. Adv Funct Mater 24:5402–5411
Ewerlin M, Demirbas D, Brüssing F, Petracic O, Ünal AA, Valencia S, Kronast F, Zabel H (2013) Magnetic dipole and higher pole interaction on a square lattice. Phys Rev Lett 110:177209
Woinska M, Szczytko J, Majhofer A, Gosk J, Dziatkowski K, Twardowski A (2013) Magnetic interactions in an ensemble of cubic nanoparticles: a Monte Carlo study. Phys Rev B 88:144421
Xu ST, Ma YQ, Xu YF, Sun X, Geng BQ, Zheng GH, Dai ZX (2015) Pure dipolar-interacted CoFe2O4 nanoparticles and their magnetic properties. Mater Res Bull 62:142–147
Zan FL, Ma YQ, Ma Q, Xu YF, Dai ZX, Zheng GH, Wu MZ, Li G (2013) Magnetic and impedance properties of nanocomposite CoFe2O4/Co0.7Fe0.3 and single phase CoFe2O4 via One-step hydrothermal. J Am Ceram Soc 96:3100–3107
Zan FL, Ma YQ, Ma Q, Zheng GH, Dai ZX, Wu MZ, Li G, Sun ZQ, Chen XS (2013) One-step hydrothermal synthesis and characterization of high magnetization CoFe2O4/Co0.7Fe0.3 nanocomposite permanent magnets. J Alloy Compd 553:79–85
Adeela N, Maaz K, Khan U, Karim S, Nisar A, Ahmad M, Ali G, Han XF, Duan JL, Liu J (2015) Influence of manganese substitution on structural and magnetic properties of CoFe2O4 nanoparticles. J Alloy Compd 639:533–540
Geng BQ, Ding ZL, Ma YQ (2016) Unraveling the correlation between the remanence ratio and the dipolar field in magnetic nanoparticles by tuning concentration, moment, and anisotropy. Nano Res 9(9):2772–2781
Tartaj P, González-Carreño T, Bomatí-Miguel O, Serna CJ, Bonville P (2004) Magnetic behavior of superparamagnetic Fe nanocrystals confined inside submicron-sized spherical silica particles. Phys Rev B 69:094401
Bittova B, Vejpravova JP, Morales MPG, Roca A, Mantlikova A (2012) Relaxation phenomena in ensembles of CoFe2O4 nanoparticles. J Magn Magn Mater 324:1182–1188
Mohapatra J, Mitra A, Bahadur D, Aslam M (2015) Superspin glass behavior of self-interacting CoFe2O4 nanoparticles. J Alloy Compd 628:416–423
Soares JM, Cabral FAO, Araújo JHD, Machado FLA (2011) Exchange-spring behavior in nanopowders of CoFe2O4-CoFe2. Appl Phys Lett 98:072502
Fu JC, Zhang JL, Peng Y, Zhao JG, Tan GG, Mellors NJ, Xie EQ, Han WH (2014) Unique magnetic properties and magnetization reversal process of CoFe2O4 nanotubes fabricated by electrospinning. Nanoscale 4:3932–3936
Saidani M, Belkacem W, Bezergheanu A, Cizmas CB, Mliki N (2015) Surface and interparticle interactions effects on nano-cobalt ferrites. J Alloy Compd 653:513–522
Sun X, Ma YQ, Xu YF, Xu ST, Geng BQ, Dai ZX, Zheng GH (2015) Improved magnetic performance at low and high temperatures in non-exchange-coupling CoFe2O4/CoFe2 nanocomposites. J Alloy Compd 645:51–56
Quesada A, Rubio-Marcos F, Marco JF, Mompean FJ, García-Hernández M, Fernández JF (2014) On the origin of remanence enhancement in exchange-uncoupled CoFe2O4-based composites. Appl Phys Lett 105:202405
Xu ST, Ma YQ, Zheng GH, Dai ZX (2015) Simultaneous effects of surface spins: rarely large coercivity, high remanence magnetization and jumps in the hysteresis loops observed in CoFe2O4 nanoparticles. Nanoscale 7:6520–6526
Huber DL (2005) Synthesis, properties, and applications of iron nanoparticles. Small 5:482–501
Bhowmik RN, Vasanthi V, Poddar A (2013) Alloying of Fe3O4 and Co3O4 to develop Co3xFe3(1−x)O4 ferrite with high magnetic squareness, tunable ferromagnetic parameters, and exchange bias. J Alloy Compd 578:585–594
Kneller EF, Hawig R (1991) The exchange-spring magnet: a new material principle for permanent magnets. IEEE Trans Magnetics 27: 3588–3560
Cullity BD (1972) Introduction to magnetic materials. Addison-Wesely Publishing, Reading
Lakshmi N, Bhargava H, Venugopalan OPK (2009) Magnetic properties resulting from core-shell interactions in nanosized Ni0.25Co0.25Zn0.5Fe2O4. Phys Rev B 80:174425
Bianco LD, Fiorani D, Testa AM, Bonetti E, Savini L, Signoretti S (2002) Magnetothermal behavior of a nanoscale Fe/Fe oxide granular system. Phys Rev B 66:174418
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos. 51471001 and 11174004).
Authors’ Contributions
The manuscript was written through the contributions of all authors. The experiments and characterization were performed by STX, BQG, XS and MW. All of the tests were measured by STX. YQM contributed through research guidance, discussion, and manuscript modifications. All authors read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Xu, S., Ma, Y., Geng, B. et al. The remanence ratio in CoFe2O4 nanoparticles with approximate single-domain sizes. Nanoscale Res Lett 11, 471 (2016). https://doi.org/10.1186/s11671-016-1691-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11671-016-1691-3