Abstract
Natural minerals are widely used in treatment technologies as mineral fertilizer, food additive in animal husbandry, and cosmetics because they combine valuable ion-exchanging and adsorption properties together with unique physicochemical and medical properties. Saponite (saponite clay) of the Ukrainian Podillya refers to the class of bentonites, a subclass of layered magnesium silicate montmorillonite. Clinoptilolits are aluminosilicates with carcase structure. In our work, we have coated biopolymer chitosan on the surfaces of natural minerals of Ukrainian origin — Podilsky saponite and Sokyrnitsky clinoptilolite. Chitosan mineral composites have been obtained by crosslinking of adsorbed biopolymer on saponite and clinoptilolite surface with glutaraldehyde. The obtained composites have been characterized by the physicochemical methods such as thermogravimetric/differential thermal analyses (DTA, DTG, TG), differential scanning calorimetry, mass analysis, nitrogen adsorption/desorption isotherms, scanning electron microscopy (SEM), and Fourier transform infrared (FTIR) spectroscopy to determine possible interactions between the silica and chitosan molecule. The adsorption of microquantities of cations Cu(II), Zn(II), Fe(III), Cd(II), and Pb(II) by the obtained composites and the initial natural minerals has been studied from aqueous solutions. The sorption capacities and kinetic adsorption characteristics of the adsorbents were estimated. It was found that the obtained results have shown that the ability of chitosan to coordinate heavy metal ions Zn(II), Cu(II), Cd(II), and Fe(III) is less or equal to the ability to retain ions of these metals in the pores of minerals without forming chemical bonds.
Similar content being viewed by others
Background
Application of chitinous products in wastewater treatment has received considerable attention in recent years in the literature [1–8]. In particular, the development of chitosan-based materials as useful adsorbent polymeric matrices is an expanding field in the area of adsorption science [9]. Chitosan is a type of natural polyaminosaccharide, obtained by deacetylation of chitin [10], which is a polysaccharide consisting predominantly of unbranched chains of β-(1→4)-2-acetoamido-2-deoxy-D-glucose [11]. Composites based on chitosan are economically feasible because they are easy to prepare and involve inexpensive chemical reagents [11]. Recently, chitosan composites have been developed to adsorb heavy metals and dyes from wastewater [10, 12–15].
Chitosan composites have been proven to have better adsorption capacity and resistance to acidic environment [11]. Various methods of preparation of hybrid materials based on inorganic materials and polysaccharides such as chitin [1–8] and chitosan for different applications have been studied [9, 11, 16–18]. Different kinds of substances have been used to form composite with chitosan such as silica, montmorillonite, polyurethane, activated clay, bentonite, polyvinyl alcohol, polyvinyl chloride, kaolinite, oil palm ash, perlite, and magnetite [19–23]. Although such minerals possess high adsorption capabilities, the modification of their structure can successfully improve their capabilities. In work [24], chitosan/attapulgite composites are applied as an adsorbent for the removal of chromium and iron ions from aqueous solution of both single and binary systems. Attapulgite is a hydrated octahedral-layered magnesium aluminum silicate mineral with large surface area, excellent chemical stability, and strong adsorption. Equilibrium data were well described by the Freundlich isotherm models, indicating multilayer adsorption for Cr(III) and Fe(III) onto composites. Kinetic experiments showed that composites offered fast kinetics for adsorption of Cr(III) and Fe(III), and the diffusion-controlled process as the essential adsorption rate-controlling step was also proposed. Moreover, the initial adsorption rates of Cr(III) were faster than that of Fe(III) with the increase of temperature and initial concentrations. The thermodynamic analysis presented the endothermic, spontaneous, and entropy gained nature of the process [24].
The removal of nickel (II) from the aqueous solutions through adsorption on to biopolymer sorbents, such as calcium alginate, chitosan-coated calcium alginate, and chitosan-coated silica, was studied using equilibrium batch and column flow techniques. According to the study, the maximum monolayer adsorption capacity of calcium alginate, chitosan-coated calcium alginate, and chitosan-coated silica, as obtained from the Langmuir adsorption isotherm, was found to be 310.4, 222.2, and 254.3 mg/g, respectively [25].
Polymer/montmorillonite nanocomposites have improved properties such as excellent mechanical properties, thermal stability, gas barrier, and flame retardation in comparison to conventional composites. The isomorphous substitutions of Al3+ for Si4+ in the tetrahedral layer and Mg2+ for Al3+ in the octahedral layer have resulted in a negatively charged surface on montmorillonite. With these structural characteristics, montmorillonite has excellent sorption properties and possesses available sorption sites within its interlayer space as well as large surface area and more narrow channels inside. Produced chitosan coated montmorillonite for the removal of Cr(VI) [11].
This work describes the synthesis of the composite material based on chitosan and natural minerals clinoptilolite and saponite, for their use as a biosorbents. Obtained composites were characterized by physicochemical methods, such as thermal analysis and textural properties. Adsorption properties of the obtained hybrid material were studied with respect to highly toxic heavy metals: cadmium(II), lead(II), copper(II), zinc(II), and iron(III), which are common contaminants of industrial wastewaters. Conditions connected with the optimum pH value of the medium, interaction time, and adsorption capacity were studied.
Experimental part
Materials
Sokyrnitskiy clinoptilolite of Ukrainian Zakarpattya has the general formula (Ca,Na,K2)Al2Si7O18·6H2O, chemical content (in mass %): SiO2—76.07; Al2O3—12.4; K2O—2.80; CaO—2.09; Na2O—2.05; Fe2O3—0.90; FeO—0.76; TiO2—0.19; P2O5—0.12; MgO—0.07; MnO—0.07; SO3—0.08. Saponite of Ukrainian Podillya has the general formula (Ca0.5,Na)0.33(Mg,Fe)3(Si,Al)4O10(OH)2·4H2O. Chitosan is originally from shrimps, Sigma-Aldrich, No. 417963, molecular weight from 190,000 to 370,000 Da, degree of deacetylation — not less than 75%, and solubility 10 mg/ml. All chemicals are purchased from Sigma-Aldrich were of reagent grade.
Methods
-
Composites chitosan-saponite and chitosan-clinoptilolite were obtained by impregnation 20 g of minerals (saponite and clinoptilolite) by 285 ml of chitosan solution with a concentration of 7 mg/ml in acetic acid (pH 2.6). The mixture was put in flat-bottom flask and mixed by the magnetic stirrer MM-5 for 2 h. The obtained substance was dried at 50 °C. The obtained composites were placed in 12.5 ml of 0.25% solution of glutaraldehyde in water and heated at 50 °C for 2 h. Such quantity of glutaraldehyde is proper for crosslinking of 5% of accessible amino groups of polymer. The crosslinked chitosan on the surface of the minerals were washed with distilled water and dried at 50 °C. Thus, based on the theoretical mass ratio, the obtained organic and mineral components of the composite was chitosan:silica = 1:10 [15].
-
Buffer solutions with pH 1.0 prepared from the standard titrimetric substance of HCl acid, pH 2.5, and 5.0 from glacial acetic acid, and pH 8.0 were prepared from 17 ml of 1 M acetic acid and 5 ml of 25% ammonia solution and adding distilled water up to 1 l. The pH values of all buffer solutions were controlled by a pH meter.
-
FTIR spectra of the samples of the initial chitosan and reaction products were recorded using an IR spectrometer with Fourier transformation (Thermo Nicolet Nexus FT-IR, USA). For this purpose, the samples were ground in an agate mortar and pressed with KBr.
-
Thermal analysis. Thermal analysis was carried out on a STA 449 Jupiter F1, Netzsch (Germany) under the following operational conditions: heating rate of 10 °C min−1, a dynamic atmosphere of synthetic air (50 ml min−1), temperature range of 30–950 °C, sample mass ~18 mg, and sensor thermocouple type S TG-DSC. As a reference, empty Al2O3 crucible was used. The gaseous products emitted during decomposition of materials were analyzed by FTIR spectrometer Brucker (Germany) and by QMS 403C Aeölos (Germany) coupling online to the STA instrument. The QMS data were gathered in the range of from 10 to 160 amu. The FTIR spectra were recorded in the spectral range of 600–4000 cm−1 with 16 scans per spectrum at a resolution of 4 cm−1.
-
Surface area and average pore diameter analysis. The specific surface area and the average pore diameter of the composite were determined with the BET instrument ASAP 2405 (Micromeritics Instrument Co., USA). The isotherm plots were used to calculate the specific surface area and the average pore diameter of chitosan-silica composite.
-
Surface morphology analysis. The surface morphology of chitosan–silica composite was observed by using a scanning electron microscope (SEM, LEO 1430VP, Carl Zeiss, Germany).
The investigations of adsorption properties of the obtained composite with respect to zinc, copper, cadmium, lead, and iron were carried out in the static mode with periodic hand-stirring. For that, the sample of 0.1 g of synthesized adsorbent was contacted with 25 ml of solutions at different concentrations of salts: Zn(NO3)2·6H2O, CuCl2·2H2O, Cd(NO3)2·4H2O, Pb(NO3)2, FeCl3, which were prepared according to [26]. Determination of the equilibrium concentration of the metals was carried out by atomic absorption using a flaming atomic absorption spectrophotometer “Saturn” (Ukraine) in a “air-propane-butane” flame mixture.
Calculations
The adsorption capacity (q e ) was calculated using the formula:
the degree of adsorption (R) was calculated using the formula:
where c 0 is the concentration of initial solution, c e is the equilibrium concentration of metal, V is the volume of equilibrium solution, and m is the mass of adsorbent.
Results and Discussion
Physicochemical Characteristics of the Composite
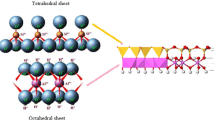
Chitosan has a high affinity to the surface of silica-based minerals due to the interaction between part of protonated amino groups of polymer and dissociated hydroxyl groups of silica, which are formed in aqueous solution [15]. Thus, the mechanism of the chitosan interaction with the selected minerals is due to the electrostatic interaction as well as hydrogen binding. The scheme of structure of chitosan mineral composites is presented in Fig. 1.
In order to ascertain the immobilization of chitosan onto the surface of minerals, FTIR spectroscopy was employed to characterize initial chitosan, clinoptilolite, saponite, and synthesized composites (Fig. 2). In the FTIR spectrum of chitosan (Fig. 2 (1)), the band at 3429 cm−1 corresponds to the stretching vibrations O–H of hydroxyl groups bound with carbon atoms. Intensive absorption bands at 2800–3000 cm−1 are observed due to the C–H stretching vibrations. The band at 1580 cm−1 corresponds to the deformation vibrations of –NH2, 1420 and 1380 cm−1 for C–H binding vibrations, 1310 cm−1 for asymmetric C–O–C stretching vibrations, and 1080 cm−1 for C–O stretching vibration of CH–OH.
The FTIR spectrum of the synthesized composites (Fig. 2 (4 and 5)) has shown a shift of the band 1530 cm-1 of –NH2 deformation vibrations in comparison with the spectrum of the initial chitosan. An intensive absorbance at 1090 and 1000 cm−1 represents the Si–O stretching vibrations. Absorbance band at 610 and 660 cm−1 represents to stretching vibrations of Si–O and shifted in comparison with the FTIR-spectra of initial minerals. Absorbance bands at 556 and 463 cm−1 and 518 and 466 cm−1 represent the deformation vibrations of Al–O–Si and Si–O–Si in chitosan-clinoptilolite and chitosan-saponite, respectively. It was observed that the characteristic bands at 1633 and 1645 cm−1 at the FTIR-spectra of chitosan-clinoptilolite and chitosan-saponite, respectively, describe azomethine bonds C=N, formed after glutaraldehyde treatment [27].
The influence of polymeric coating on thermal properties of mineral surfaces was studied by conducting DSC-MS analysis. Applying of these methods of investigations were also conducted in order to determine the mass ratio of chitosan coating on the mineral surfaces.
For the TG-curve of chitosan (Fig. 3a), two decomposition temperatures can be found. The initial weight loss of 11% from room temperature (30 °C) up to 190 °C corresponds to the release of adsorbed water [21]. The second recorded decomposition region (190–1000 °C) completely applies to the weight loss of chitosan. Figure 3b, c presents the TG, DTG, and DSC curves of pure clinoptilolite and saponite.
Comparing the thermogravimetric curves of chitosan-clinoptilolite and chitosan-saponite composites (with the curves of the initial chitosan and pure minerals; Fig. 3d, e), one could observe that the maximum of each decomposition region of composites was observed at lower temperatures than for similar process of the pure minerals. For instance, coated clinoptilolite and saponite begin lost the water at T max 123 and 108 °C, when pure minerals – at 159 and 145 °C, respectively (Table 1). The main decomposition of the composite materials occurred at T max 280 and 277 °C when pure clinoptilolite and saponite did not show decomposition at this temperatures, in contrast with the native chitosan, which is characterized by loss of more than 50% at T max 295 °C. Thus, the coated minerals are able to lose water faster than pure minerals and the temperature of the decomposition of polymer in composition of hybrid materials decreased by 15 °C (5 %) for chitosan-clinoptilolite composite and by 18 °C (6 %) for chitosan-saponite composite.
Comparing the results of thermogravimetric analysis for the initial and obtained composites, it was confirmed that all involved polymers to the reaction were successfully introduced to the hybrid materials. Thus, each composite contains 10 % polymer and 90 % of the mineral part (91 mg/g of chitosan).
Figure 4 presents the nitrogen adsorption/desorption isotherms measured at 77 K for the initial minerals and coated minerals by chitosan. The shape of the isotherm corresponds to the Langmuir isotherm, type II of the International Union of Pure and Applied Chemistry (IUPAC) classification. This type of isotherm commonly observed in nonporous or macroporous materials of which the steep increase of adsorbed quantity at low relative pressure indicates the presence of unrestricted monolayer and multilayer adsorption. It is seen from the isotherms that the monolayer coverage completed at the relative pressure ranges up to 0.45. The shape of the isotherms confirms prevalent presence of cylindrical pores. According to the results of surface area analysis, the pure clinoptilolite and saponite has the BET surface area 22 and 41 m2/g, respectively, which was decreased with modification of its surfaces by polymer up to 5 and 10 m2/g for partially crosslinked chitosan-clinoptilolite and partially crosslinked chitosan-saponite. The presence of mesopores and macropores is confirmed by the diagram of pore size distribution for the initial and modified minerals (Fig. 5), which was obtained by the adsorption branch of the isotherm using the BJH method. The SEM images showed uniform coating of the surface of the minerals by chitosan (Figs. 6 and 7).
Influence of pH on Adsorption
Montmorillonites and clays are perspective ion-exchangers; however, it is necessary to study the ability of those minerals to adsorb cationic forms of heavy metals, which is presented in natural waters and wastewaters. It is crucial to study factors which could influence the sorption behavior. For instance, the medium acidity is a very important factor because its plain main role on ionic form of the metals in aqueous solutions. In an acidic medium created by hydrochloric or acetic acid, metals such Cu(II), Zn(II), Fe(III), Cd(II), and Pb(II) could be present in a form of chlorides and acetates. Investigation of sorption properties of the synthesized composite began with the determination of medium acidity for the highest removal of the studied ions.
The degree of adsorption of Cu(II), Zn(II), Fe(III), Cd(II), and Pb(II) cations by composites based on partially crosslinked chitosan and natural minerals clinoptilolite and saponite as a function of the medium acidity were investigated in different chemical compositions of buffer solutions. Ionic forms of cations presented in Table 2. The obtained degree of adsorption of Cu(II), Zn(II), Fe(III), Cd(II), and Pb(II) cations by studied composites is presented in Table 3. It can be seen that the highest degree of adsorption (up to 99.00%) on the surface of the obtained composite was observed for all cations from the solutions with a concentration 4 mg/l of studied metals in the slightly basic (pH 8.0, ammonium acetate buffer) and neutral medium. In the acidic medium, the decreasing of degree of adsorption of cationic forms of studied metals was observed.
Thus, the synthesized composite showed adsorption activity with respect to the investigated ions in neutral and slightly basic medium and confirmed that the adsorption process occurs through complexation of aqua, acetic, or bi-ligand complexes of studied ions with amino groups of chitosan. The values of medium acidity, at which the maximum adsorption activities of chitosan-based composites for each of the studied ions were achieved, correspond to the published data of complexation conditions of these ions with amino groups of chitosan in solutions [28].
Influence of Contact Time on Adsorption
According to the obtained results for all studied ions presented in Table 4, the degree of adsorption consistently increases for several hours, but the maximum degree of adsorption of all studied ions by the composites surface is achieved for a day which is typical of polymeric adsorbents where the sorption characteristics are defined by interactions between the ions and the functional groups of supported chitosan, which indirectly confirm that the adsorption process occurred by chitosan.
Influence of the Initial Metal Ion Concentration on Adsorption
Adsorption isotherms in the static mode for each ion were obtained for calculation of the values of the adsorption capacity of the composite and compare with the values for the initial minerals. The study of influence of initial metal ion concentration on adsorption in neutral medium has shown that adsorption capacity of coated clinoptilolite and saponite by chitosan in case of Pb(II) aqua ions reached more higher values than for pure minerals (Figs. 8, 9, 10, and 11). The values of adsorption capacity of chitosan-clinoptilolite and chitosan-saponite composites compared to pure minerals presented in Table 5. Obtained results has shown that the ability of chitosan to coordinate heavy metal ions Zn(II), Cu(II), Cd(II), and Fe(III) is less or equal to the ability to retain ions of these metals in the pores of minerals without forming chemical bonds.
Conclusions
An investigation of properties of coated minerals of Ukrainian origin clinoptilolite and saponite by biopolymer chitosan has shown the number of advantages of obtained materials from the side of their physical-chemical properties. It was found that the synthesized composites contain the best characteristics of the initial materials: high biocompatibility and complexation ability of functional groups of chitosan and low cost and environmental friendliness of minerals clinoptilolite and saponite. The study of complexation properties of coated minerals by chitosan has shown that the ability of chitosan to coordinate heavy metal ions Zn(II), Cu(II), Cd(II), and Fe(III) is less or equal to the ability to retain ions of these metals in the pores of minerals without forming chemical bonds.
References
Li CB, Hein S, Wang K (2008) Biosorption of chitin and chitosan. Mater Sci Technol 24(9):1089–1100
Wysokowski M, Petrenko I, Stelling AL, Stawski D, Jesionowski T, Ehrlich H (2015) Poriferan chitin as a versatile template for extreme biomimetics. Polymer 7(2):235–265
Liu D, Zhu Y, Li Z, Tian D, Chen L, Chen P (2013) Chitin nanofibrils for rapid and efficient removal of metal ions from water system. Carbohydr Polym 98:483–489
Kousalya GN, Gandhi MR, Viswanathan N, Meenakshi S (2010) Preparation and metal uptake studies of modified forms of chitin. Int J Biol Macromol 47:583–589
Kim S-H, Song H, Nisola GM, Ahn J, Galera MM, Lee C, Chung W-J (2006) Adsorption of lead(II) ions using surface-modified chitins. J Ind Eng Chem 12:469–475
Pigatto G, Lodi A, El F, Palma MSA, Convertia A (2013) Chitin as biosorbent for phenol removal from aqueous solution: equilibrium, kinetic and thermodynamic studies. Chem Eng Process 70:131–139
Wysokowski M, Klapiszewski Ł, Moszyński D, Bartczak P, Szatkowski T, Majchrzak I, Siwińska-Stefańska K, Bazhenov VV, Jesionowski T (2014) Modification of chitin with kraft lignin and development of new biosorbents for removal of cadmium(II) and nickel(II) ions. Mar Drugs 12(4):2245–2268
Klapiszewski Ł, Wysokowski M, Majchrzak I, Szatkowski T, Nowacka M, Siwińska-Stefańska K, Szwarc-Rzepka K, Bartczak P, Ehrlich H, Jesionowski T (2013) Preparation and characterization of multifunctional chitin/lignin materials. Journal of Nanomaterials. 2013;2013:1–13.
Crini G, Badot PM (2008) Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solutions by adsorption processes using batch studies: a review of recent literature. Prog Polym Sci 33:399–447
Budnyak TM, Tertykh VA, Yanovska ES (2013) Chitosan and its derivatives as sorbents for effective removal of metal ions: review. Surface 5(Suppl 20):118–134
Wan Ngah WS, Teong LC, Hanafiah Ma KM (2011) Adsorption of dyes and heavy metal ions by chitosan composites: a review. Carbohydr Polym 83:1446–1456
Budnyak TM, Tertykh VA, Yanovska ES, Kołodynska D, Bartyzel A (2015) Adsorption of V(V), Mo(VI) and Cr(VI) oxoanions by chitosan-silica composite synthesized by Mannich reaction. Adsorpt Sci Technol 6–8:645–657
Budnyak T, Yanovska E, Ischenko M, Tertykh V (2014) Adsorption of heavy metals by chitosan crosslinked with glutaraldehyde. Visnyk of KNU. Chemistry 1:35–38
Budnyak TM, Pylypchuk IV, Tertykh VA, Yanovska ES, Kolodynska D (2015) Synthesis and adsorption properties of chitosan-silica nanocomposite prepared by sol-gel method. Nanoscale Res Lett 87:1–10
Budnyak T, Tertykh V, Yanovska E (2014) Chitosan immobilized on silica surface for wastewater treatment. Mater Sci (Medžiagotyra) 20(2):177–182
Darder M, Aranda P, Ruiz-hitzky E Chitosan-Clay Bio-Nanocomposites. In: Avérous L, Pollet E, editors. Environmental silicate nano-biocomposites. Green Energy and Technology. Springer-Verlag, London, 2012. p. 365–391
Jiuhui QU (2008) Research progress of novel adsorption processes in water purification: a review. J Environ Sci (China) 20:1–13
Muzzarelli R (2011) Potential of chitin/chitosan-bearing materials for uranium recovery: an interdisciplinary review. Carbohydr Polym 84:54–63
Kołodyńska D, Gęca M, Pylypchuk IV, Hubicki Z (2016) Development of new effective sorbents based on nanomagnetite. Nanoscale Res Lett 152:1–11
Pylypchuk IV, Kołodyńska D, Kozioł M, Gorbyk PP (2016) Gd-DTPA adsorption on chitosan/magnetite nanocomposites. Nanoscale Res Lett 168:1–11
Budnyak TM, Yanovska ES, Kołodyńska D, Sternik D, Pylypchuk IV, Ischenko MV, Tertykh VA (2016) Preparation and properties of organomineral adsorbent obtained by sol–gel technology. J Therm Anal Calorim 125:1335–1351
Budnyak TM, Strizhak AV, Gładysz-Płaska A, Sternik D, Komarov IV, Kołodyńska D, Majdan M, Tertykh VA (2016) Silica with immobilized phosphinic acid-derivative for uranium extraction. J Hazard Mater 314:326–340
Gorbyk PP, Lerman LB, Petranovska AL, Turanska SP, Pylypchuk IV Magnetosensitive nanocomposites with hierarchical nanoarchitecture as biomedical nanorobots: synthesis, properties, and application. In: Grumezescu AM, editor. Fabrication and Self-Assembly of Nanobiomaterials: Applications of Nanobiomaterials. Elsevier; 2016. p. 289–332.
Zou X, Pan J, Ou H, Wang X, Guan W, Li C, Yana Y, Duanc Y (2011) Adsorptive removal of Cr(III) and Fe(III) from aqueous solution by chitosan/attapulgite composites: equilibrium, thermodynamics and kinetics. Chem Eng J 167:112–121
Vijaya Y, Popuri SR, Boddu VM, Krishnaiah A (2008) Modified chitosan and calcium alginate biopolymer sorbents for removal of nickel (II) through adsorption. Carbohydr Polym 72:261–271
Marchenko Z, Balcerzak M (1998) Spectrofotometry Metods in Inorganic Analisys. Warsaw: Naukove, Naukowe PWN; (in Polish).
Wan Ngah WS, Teong LC, Wong CS, Hanafiah MAKM (2012) Preparation and characterization of chitosan-zeolite composites. J Appl Polym Sci 125:2417–2425
Guibal E, Milot C, Tobin J (1998) Metal–anion sorption by chitosan beads: equilibrium a kinetic studies. Ind Eng Chem Res 37(Suppl 4):1454–1463
Acknowledgements
The research leading to these results is financed by the People Programme (Marie Curie Actions) of the European Union’s Seventh Framework Programme FP7/2007-2013/ under REA grant agreement No. PIRSES-GA-2013-612484.
Authors’ contributions
TB carried out the whole study, realized the synthetic part, explained and discussed obtained results, and prepared the manuscript. EY coordinated the analytical part of the study. OK conducted the analytical measurements. DS carried out the physical-chemical measurements of the investigated materials. VT designed and coordinated the study. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at http://dx.doi.org/10.1186/s11671-016-1752-7.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Budnyak, T.M., Yanovska, E.S., Kichkiruk, O.Y. et al. Natural Minerals Coated by Biopolymer Chitosan: Synthesis, Physicochemical, and Adsorption Properties. Nanoscale Res Lett 11, 492 (2016). https://doi.org/10.1186/s11671-016-1696-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11671-016-1696-y