Abstract
Nanometer-sized titanium dioxide (TiO2) is an environmentally friendly optical semiconductor material. It has wide application value in many fields due to its excellent structural, optical, and chemical properties. The photocatalytic process of nano-TiO2 converts light energy into electrical or chemical energy under mild conditions. In recent years, the study and application of nano-TiO2 in the agricultural sector has gradually attracted attention. The nano-TiO2 applications of degrading pesticides, plant germination and growth, crop disease control, water purification, pesticide residue detection, etc. are good prospects. This review describes all of these applications and the research status and development, including the underlying principles, features, comprehensive applications, functional modification, and potential future directions, for TiO2 in agriculture.
Similar content being viewed by others
Review
Introduction and Background
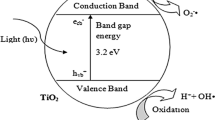
To date, the photo semiconductor, titanium dioxide (TiO2), has been proven to be the most effective and useful photocatalyst for both fundamental research and practical applications due to its high-efficiency, photochemical stability, nontoxic nature, and low cost [1–4]. Much research has been performed to explore the photocatalytic activity of TiO2 photo semiconductors in their nano form [5–7]. The photocatalytic activity mechanism of nano-TiO2 has been extensively studied in the literature [8–13], and the basic photocatalytic mechanism is shown in Fig. 1. Upon absorption of light energy larger than the band gap of TiO2, electrons are excited from the valence band to the conduction band, which creates electron (e−)–hole (h+) pairs. These charge carriers can rapidly migrate to the surfaces of catalyst particles, where they are ultimately trapped and undergo redox chemistry with suitable substrates. Thus, the trapped hole can react with chemisorbed OH− or H2O to produce the OH· radicals. Oxygen, which is present in the system, acts as an efficient electron scavenger. Additionally, any other oxidant, such as OH−, can trap electrons [14–16].
Spatial distribution of photogenerated charge carriers in TiO2 [16]
Nano-TiO2 photo semiconductors have many applications in many fields including photocatalysis, agriculture, dye-sensitized solar cells, and biomedical devices [17]. However, in the agricultural field, the use of TiO2 nanomaterials is relatively new and requires further exploration. The nano-TiO2 photo semiconductor continues to attract attention of agricultural researchers because of its favorable physical/chemical properties, low cost, availability, and high stability. Thus, nano-TiO2 photo semiconductors have many application possibilities in agriculture including degradation of pesticides, plant protection, and residue detection. However, one disadvantage of TiO2 nanomaterials is that they are mostly active in the presence of UV light due to their large band gap of approximately 3.2 eV [18, 19]. The UV regime is only a small fraction of the Sun’s energy (<10%) [20]. Therefore, this property limits the application of TiO2 nanomaterials in agriculture, and the highly efficient use of TiO2 nanomaterials is sometimes prevented. Thus, several approaches have been developed to alleviate this problem and to improve the photocatalytic activity of TiO2 nanomaterials for a wide range of applications. One effective method for improving the performance of TiO2 nanomaterials is to increase their optical activity by shifting the response onset from the UV to the visible region by doping the TiO2 nanomaterial with different metals or other elements [21].
This paper aims to review and summarize the recent applications and research on nano-TiO2 photo semiconductors and their doping complexes in agriculture. The topics include pesticide degradation, plant germination and growth, crop disease control, water purification, and pesticide residue detection.
Application of TiO2 Photo Semiconductors in Agriculture
Pesticide Degradation
Pesticides are widely used in agriculture, although their excessive usage may create hazards to both humans and the environment. Repeated use of pesticides results in a frequent occurrence of residues in the environment and in biota. Most pesticide residues require effective treatment and further removal due to their toxicity, high chemical stability, and low biodegradability. Consequently, considerable efforts have been devoted to developing methods that can remove residual pesticides and destroy bio-recalcitrant organic contaminants [22]. Semiconductor photocatalysis is a promising approach to remedy the pesticide residue problem, and it has attracted significant attention [14, 23]. TiO2 is the most investigated photo semiconductor. For the past decade, it has been widely studied as an efficient photocatalyst for pesticides [14].
In the photocatalytic oxidation process, pesticides are destroyed in the presence of TiO2 photocatalysts and a UV light source. As illustrated in Fig. 2, when TiO2 is irradiated with photons whose energy is equal to or greater than its band gap energy (Eg = 3.2 eV), electron–hole pairs are created. In an aqueous system, these holes react with H2O or OH− that are adsorbed on the semiconductor surface to produce OH· radicals, which are the strongest oxidants in this process [24–27]. These radicals react with pesticides that are adsorbed on the surface and decompose the pesticides [20]. The pesticides are degraded into H2O, CO2, and other biologically degradable and less toxic substances without secondary pollution. Rabindranathan reported that the TiO2 photocatalyst is effective in degrading the phosphamidon insecticide [28]. Several factors (e.g., phosphamidon concentration, pH of the system, catalyst loading, and the presence of anions) influence the degradation rate [28]. Lhomme reported the photocatalytic degradation of chlortoluron and cyproconazole pesticides on TiO2-coated media, and the process was found to be effective in degrading and mineralizing the pesticides [29]. Recent studies have found that the TiO2 morphology plays a key role on the photocatalytic activity. As shown in Fig. 3, TiO2 nanotube length has significant effect on the photocatalytic degradation of paraquat [30], and short tubes with a small internal diameter exhibit poor photocatalytic activity because pollutant diffusion is ineffective. The result shows that the optimal activity was found for 7-μm-long TiO2 tubes, and tubes longer than 7 μm have thinner walls; thus, light is absorbed on a longer distance and pollutant has to diffuse further to reach the oxidizing species [30].
Schematic of the pesticide degradation mechanism: photocatalytic oxidation of TiO2 [24]
SEM analysis—side and top view for various tube lengths: 1.5 μm (a, d), 7 μm (b, e), and 10 μm (c, f) [30]
This TiO2 photocatalytic degradation process of pesticides mainly relies on the in situ generation of highly reactive OH· radicals, which are capable of converting pesticides into relatively innocuous end products. However, the limitations for the wide application of TiO2 semiconductors for pesticide photocatalytic degradation include the high rate of electron–hole recombination, wide band gap, and inefficient visible light harvesting catalysts [31, 32]. Minimization of the electron–hole recombination and efficient visible light excitation are the major issues that increase the photocatalytic efficiency for pesticide degradation. To overcome these problems, many modifications have been applied to TiO2 nanomaterials such as doping with a metal coating, surface sensitization, surface area increase or designing, and developing secondary mixed oxides [33, 34]. The modifications of TiO2 nanomaterials with different special modifiers, such as Ag+, WO3, and W, have enhanced the pesticide photocatalytic degradation properties of the catalyst [35, 36]. An efficient charge separation can be obtained by coupling two semiconductor particles that have different energy levels [34, 37]. Figure 4 presents WO3 doping as an example. WO3 (Eg = 2.8 eV) can function as an electron accepting species in the presence of visible light, which is favorable for producing electron–hole pairs and for improving the pesticide photocatalytic degradation [34].
Solar photocatalytic activity of TiO2 modified with WO3 for the organophosphorus pesticide degradation [34]
A substantial amount of research has been focused on modifying the TiO2 photocatalyst for enhancing pesticide degradation [22]. Ramos-Delgado et al. reported the solar photocatalytic activity of TiO2, which was modified with WO3, for degrading the organophosphorus pesticide [34]. The TiO2 semiconductor, which was loaded with 2% WO3, exhibited better solar photocatalytic behavior for degrading the malathion pesticide compared with bare TiO2. This was attributed to the formation of smaller clusters and a higher surface area, which reduced the electron–hole recombination process and resulted in a better contact area between the catalyst particles and the pesticide. Thus, the photocatalytic reactivity and efficiency were improved [34]. Guan et al. reported a W/TiO2 catalyst that was constructed for the photocatalytic degradation and mineralization of avermectin insecticide microcapsules. The catalyst had the highest photocatalytic activity with a 4.0 mol% W-doped amount due to the presence of electron-trapping centers (W6+) in W-doped TiO2 solid solutions. Guan et al. reported different types of TiO2-based photodegradable nano-imidacloprid insecticides [14]. Photocatalysts, including TiO2, sodium dodecyl sulfate (SDS)/TiO2, Ag/TiO2, and SDS/Ag/TiO2, were constructed for the photocatalytic degradation of the nano-imidacloprid insecticide. The photocatalytic activity of SDS/Ag/TiO2 was the highest among all of the photocatalysts due to its large specific surface area compared with TiO2, which led to the fast adsorption of reactants and enrichment of the insecticide. Moreover, depositing silver in the SDS/Ag/TiO2 photocatalyst significantly promotes the photocatalytic activity.
Plant Germination and Growth
In recent years, various researchers have studied the effects of nanomaterials on plant germination and growth to promote the use of nanomaterials for agricultural applications [38]. TiO2 nanomaterials can induce active oxygen, including superoxide and hydroxide anions, in the photocatalytic process, which increases the seed stress resistance and water and oxygen intake. These are required for the fast germination of plants. Zheng et al. reported the effects of TiO2 photocatalysts on the growth of spinach seeds. They demonstrated that the nano-TiO2-treated seeds that were produced from plants that had a higher dry weight, higher photosynthetic rate, and increased chlorophyll formation. This suggested that TiO2 nanomaterials promoted the absorption of inorganic nutrients and increased the photosynthetic rate [39]. The research results of Song et al. showed that the effect on plant growth was more pronounced with TiO2 nanoparticles than with bulk TiO2. TiO2 nanoparticles stimulated plant growth at low concentrations but inhibited plant growth at high concentrations [40]. Yang showed that spinach leaves could be kept green using nano-anatase TiO2 treatment due to N2 fixation. Additionally, the fresh weight, dry weight, and the content of total nitrogen, NH4 +, chlorophyll, and protein in spinach were clearly increased [41]. As shown in Fig. 5, Raliya et al. reported the physiological effects of TiO2 nanoparticles in mung bean. The results demonstrated a significant increase in plant growth for plants that were treated with TiO2 nanoparticles. In the control, plants exposed to TiO2 nanoparticles showed significant improvements in shoot length, root length, root area, and root nodules [42].
Phenology of a mung bean plant under various treatments (control, O TiO2: ordinary titanium dioxide, n TiO2: nano titanium dioxide) [42]
Crop Disease Control
Conventional bactericidal methods that are used to protect plants against pathogens apply chemical pesticides to the irrigation water. However, this method of controlling plant diseases is hazardous both to humans and to the environment. Photochemical disinfection of plant pathogens using TiO2 thin films offers an alternative method for preventing plant pathogens [43]. The TiO2 photocatalyst technique has a potential for agricultural applications because it does not form dangerous compounds [44]. Under light, TiO2 nanomaterials generate superoxide ion radicals and hydroxides. These active oxygen species are effective antimicrobial agents. In recent years, various researchers have studied the effect of nano-TiO2 photo semiconductors in controlling crop diseases. However, UV accounts for only approximately 3% of the solar light spectrum. This limits the TiO2 photocatalytic disinfection application under visible light irradiation [44]. Yao et al. reported that the TiO2 thin film photocatalytic efficiency is improved under visible light by doping with a novel photosensitive dye. Thus, phytopathogenic bacteria in vegetable crops can be effectively inhibited by visible light irradiation. Cui et al. studied the bactericidal effect of nano-TiO2 on cucumbers [45]. The nano-TiO2 formed a successive, adhesive, and transparent film on the surface of the leaves. Further, the nano-TiO2-treated cucumber leaves had powerful bactericidal effects on plant pathogens due to the photocatalytic and photo biological effects of TiO2, which inhibited bacterial and fungal diseases.
Water Purification
In recent years, the growing concern about the problem of water decontamination from organic pollutants during agricultural production has led to research on methods that improve the efficiency and lower the consumption of chemical reagents [46]. Because photocatalysts use solar energy, the photocatalytic decomposition of organic pollutants in water is of particular interest and has received significant attention from scientists [47–50]. TiO2 is the most popular semiconductor that is used in photocatalytic processes [47–50]. TiO2 photo semiconductors that are a large size are stoichiometric and thus exhibit poor photocatalytic activity. However, nano-TiO2 crystallites (typical size <50 nm) have the expected electronic properties for applications in photocatalysis because of their higher activity [51]. In this photocatalysis process, reactive species can be formed on the surface of a nano-TiO2 photocatalyst that is exposed to UV radiation. The complete degradation and mineralization of a large variety of organic contaminants can be achieved in most cases [52, 53].
However, the main problem, which has limited the practical application of nano-TiO2 photocatalysis for water purification, is either a relatively low process rate or a limited efficiency for the use of irradiated energy [1, 54, 55]. A possible approach to solve this problem is the exploitation of low-cost radiation such as solar energy [56, 57]. However, the intensity of ultraviolet radiation in the solar spectrum is very limited. Therefore, the use of metals or metal oxide doping to extend the TiO2 absorption to the visible range is currently a good option for solving the problem. This approach enhances the photocatalytic activity of TiO2 and improves the utilization efficiency of the radiation energy [20, 58]. For instance, it was determined that the addition of fluoride to TiO2 significantly enhances the degradation rate of phenol [22, 59]. The dominant parameters (e.g., dopant nature, dopant concentration, and thermal treatment) affect the material [60]. Vione reported that fluoride addition to TiO2 enhanced the photocatalytic degradation of many organic compounds that were transforming via different pathways [46]. Bessekhouad reported that alkaline-doped TiO2 at low concentrations could be a promising material to degrade organic pollutants. The best results were obtained for 5% Li-doped TiO2 that was prepared using the impregnation technique [51]. Brezová et al. reported that the presence of metals, such as Li+, Zn2+, Cd2+, Pt0, Ce3+, Mn2+, Al3+, and Fe3+, could significantly change the photoactivity of TiO2 that was prepared using the sol–gel technique [60]. In addition, the effect of doping TiO2 with Li and Rb was studied by López et al., and the obtained materials were used to decompose 2,4-dinitroaniline [59].
Pesticide Residue Detection
Depending on their aqueous solubility, pesticides either remain in the soil or enter surface waters and ground waters. Pesticide degradation residues can remain in vegetables, animals, and water sources and can become more concentrated as they move up the food chain. There is an increasing interest in developing systems to sense, monitor, and remove pesticide residues because they are toxic even at trace levels.
Currently, pesticide detection methods typically use liquid or gas chromatography coupled with mass spectrometric detection (HPLC-MS and GC-MS) due to the sensitivity and reliability of these techniques. However, these approaches require meticulous sample preparation and highly qualified technicians [61]. Nanomaterial-based sensors can be used to detect pesticide residues. These nanosensors are alternatives to traditional methods due to their high sensitivity, low detection limits, high selectivity, fast response, and small size. Because of their simplicity, low cost, and ease of miniaturization, electrochemical and optical biosensors are widely used for detecting pesticides.
During recent decades, nano-TiO2 photo semiconductors, which are efficient sorbents for enriching and detecting pesticides, have attracted significant attention in the photocatalytic and photoelectrochemical area due to their nontoxicity, hydrophilicity, availability, and stability against photocorrosion. Additionally, they have a suitable flat band potential and are easily supported on various substrates [62–65].
TiO2 was used as an efficient and selective sorbent to recognize the phosphorylation moiety based on a strong chelation with phospho-moieties. The affinity of TiO2 towards the phosphoric group is favorable for fast enrichment and detection of free organophosphate pesticides [19]. However, the wide band gap of TiO2 (∼3.2 eV, anatase) allows it to absorb only the ultraviolet light (<387 nm). To extend its photo response to the visible region and to promote the photoelectric conversion efficiency, many modification methods have been applied (e.g., dye sensitization, metal ion/nonmetal atom doping, semiconductor coupling, and noble metal deposition) [19, 66]. Of the abovementioned methods, by considering the high electron mobility of nanocrystals and the possibility of shifting the optical band gap to the visible light region using organic materials, the organic–inorganic heterojunction can produce a robust photoelectrochemical sensor. Zhou reported that graphene-modified TiO2 nanotube arrays exhibit an excellent enrichment efficiency for carbamate pesticides including metolcarb, carbaryl, isoprocarb, and diethofencarb. The detection limits of these carbamate pesticides range from 2.27 to 3.26 μg L−1. The method could be used as a faster and easier alternative procedure for routine analysis of carbamate pesticides [67]. Li et al. developed two photoelectrochemical sensors to detect dichlofenthion and chlorpyrifos pesticides. The sensors were based on a TiO2 photocatalyst coupled with electrochemical detection, which is a derivative of an electrochemical sensor and sensitized TiO2 [68, 69].
Conclusions
Over the past decades, nano-TiO2 has shown its potential for agricultural applications because of its high photocatalytic disinfection and photo biological effects coupled with its low price, nontoxicity, and stable performance. The continuous breakthroughs in the synthesis and modifications of TiO2 nanomaterials have resulted in new properties and new agricultural applications including pesticide degradation, plant germination and growth, crop disease control, water purification, and pesticide residue detection with improved performance. The research demonstrates that nano-TiO2 photo semiconductors are essential for degrading organic pollutants, preventing and controlling plant diseases with an antiviral or antibacterial function, and protecting the environment. These characteristics provide new approaches for solving environmental pollution and pesticide residue problems in agriculture.
References
Guan HN, Chi DF, Yu J, Zhang SY (2011) Novel photodegradable insecticide W/TiO2/avermectin nanocomposites obtained by polyelectrolytes assembly. Colloids Surf B Biointerfaces 83(1):148–154
Liu RL, Ye HY, Xiong XP, Liu HQ (2010) Fabrication of TiO2/ZnO composite nanofibers by electrospinning and their photocatalytic property. Mater Chem Phys 121(3):432–439
Zhao Y, Jia XD, Waterhouse GIN, Wu LZ, Tung CH, O'Hare D, Zhang TR (2016) Layered double hydroxide nanostructured photocatalysts for renewable energy production. Adv Energy Mater 6(6):1501974
Yue D, Qian X, Zhao Y (2015) Photocatalytic remediation of ionic pollutant. Science Bulletin 60(21):1791–1806
Kim Y, Yang S, Jeon EH, Baik J, Kim N, Kim HS, Lee H (2016) Enhancement of photo-oxidation activities depending on structural distortion of Fe-doped TiO2 nanoparticles. Nanoscale Res Lett 11:41
Yu HJ, Zhao YF, Zhou C, Shang L, Peng Y, Cao YH, Wu LZ, Tung CH, Zhang TR (2013) Carbon quantum dots/TiO2 composites for efficient photocatalytic hydrogen evolution. J Mater Chem A 2(10):3344–3351
Fujishima A, Honda K (1972) Electrochemical photolysis of water at a semiconductor electrode. Nature 238:37–38
Turchi CS, Ollis DF (1990) Photocatalytic degradation of organic water contaminants: mechanisms involving hydroxyl radical attack. J Catal 122(1):178–192
Zhao YF, Chen GB, Bian T, Zhou C, Waterhouse GIN, Wu LZ, Tung CH, Smith LJ, O'Hare D, Zhang TR (2015) Defect-rich ultrathin ZnAl-layered double hydroxide nanosheets for efficient photoreduction of CO2 to CO with water. Adv Mater 27(47):7824–7831
Kamat PV, Meisel D (2002) Nanoparticles in advanced oxidation processes. Curr Opin Colloid Interface Sci 7(5-6):282–287
Amin SA, Pazouki M, Hosseinnia A (2009) Synthesis of TiO2–Ag nanocomposite with sol–gel method and investigation of its antibacterial activity against E. coli. Powder Technol 196(3):241–245
Zhao YF, Zhao B, Liu JJ, Chen GB, Gao R, Yao SY, Li MZ, Zhang QH, Gu L, Xie JL, Wen XD, Wu LZ, Tung CH, Ma D, Zhang TR (2016) Oxide‐modified nickel photocatalysts for the production of hydrocarbons in visible light. Angew Chem Int Ed 55(13):4215–4219
Tanaka K, Abe K, Hisanaga T (1996) Photocatalytic water treatment on immobilized TiO2 combined with ozonation. J Photochem Photobiol A Chem 101(1):85–87
Guan HN, Chi DF, Yu J, Li XC (2008) A novel photodegradable insecticide: preparation, characterization and properties evaluation of nano-Imidacloprid. Pesticide Biochemistry and Physiology 92(2):83–91
Okawa K, Suzuki K, Takeshita T, Nakano K (2005) Degradation of chemical substances using wet peroxide oxidation under mild conditions. J Hazard Mater 127(1-3):68–72
Zhang H, Chen G, Bahnemann DW (2009) Photoelectrocatalytic materials for environmental applications. J Mater Chem 19(29):5089–5121
Roy P, Berger S, Schmuki P (2011) TiO2 nanotubes: synthesis and applications. Angew Chem Int Ed 50:2904–2939
Gogos A, Knauer K, Bucheli TD (2012) Nanomaterials in plant protection and fertilization: current state, foreseen applications, and research priorities. J Agric Food Chem 60(39):9781–9792
Kumar SG, Devi LG (2011) Review on modified TiO2 photocatalysis under UV/visible light: selected results and related mechanisms on interfacial charge carrier transfer dynamics. J Phys Chem A 115(46):13211–13241
Chen XB, Mao SS (2007) Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications. Chem Rev 107(7):2891–2959
Kouloumbos VN, Tsipi DF, Hiskia AE, Nikolic D, Breemen RB (2003) Dentification of photocatalytic degradation products of diazinon in TiO2 aqueous suspensions using GC/MS/MS and LC/MS with quadrupole time-of-flight mass spectrometry. J Am Soc Mass Spectrom 14(8):803–817
Ahmed S, Rasul MG, Brown R, Hashib MA (2011) Influence of parameters on the heterogeneous photocatalytic degradation of pesticides and phenolic contaminants in wastewater. J Environ Manage 92(3):311–330
Parida KM, Sahu N, Biswal NR, Naik B, Pradhan AC (2008) Preparation, characterization, and photo-catalytic activity of sulfate-modified titania for degradation of methyl orange under visible light. J Colloid Interface Sci 318(2):231–237
Aragay G, Pino F, MerkoçI A (2012) Nanomaterials for sensing and destroying pesticides. Chem Rev 112:5317–5338.
Lee K, Ku H, Pak D (2016) OH radical generation in a photocatalytic reactor using TiO2 nanotube plates. Chemosphere 149:114–120
Devipriya S, Yesodharan S (2005) Photocatalytic degradation of pesticide contaminants in water. Sol Energy Mater Sol Cells 86(3):309–348
Szanyi J, Kwak JH (2015) Photo-catalytic oxidation of acetone on a TiO2 powder: An in situ FTIR investigation. J Molec Catalysis A: Chemi 406:213–223.
Rabindranathan S, Devipriya S, Yesodharan S (2003) Photocatalytic degradation of phosphamidon on semiconductor oxides. J Hazard Mater B 102(2-3):217–229
Lhomme L, Brosillon S, Wolbert D (2008) Photocatalytic degradation of pesticides in pure water and a commercial agricultural solution on TiO2 coated media. Chemosphere 70(3):381–386
Marien CBD, Cottineau T, Robert D, Drogui P (2016) TiO2 nanotube arrays: influence of tube length on the photocatalyticdegradation of Paraquat. Appl Catalysis B: Environ 194:1–6
Bzdon S, Goralski J, Maniukiewicz W, Perkowski J, Rogowski J, Szadkowska-Nicze M (2012) Radiation-induced synthesis of Fe-doped TiO2: characterization and catalytic properties. Radiat Phys Chem 81(3):59–63
Attarchi N, Montazer M, Toliyat T (2013) Ag/TiO2/β-CD nano composite: preparation and photo catalytic properties for methylene blue degradation. Appl Catal A Gen 467:107–116
Zhang X, Wu F, Wang Z, Guo Y, Deng N (2009) Photocatalytic degradation of 4,4′-biphenol in TiO2 suspension in the presence of cyclodextrins: a trinity integrated mechanism. J Mol Catal A Chem 301(1-2):134–139
Ramos-Delgado NA, Gracia-Pinilla MA, Maya-Trevino L, Hinojosa-Reyes L, Guzman-Mar JL, Hernández-Ramírez A (2013) Solar photocatalytic activity of TiO2 modified with WO3 on the degradation of an organophosphorus pesticide. J Hazard Mater 263P:36–44
Vamathevan V, Amal R, Beydoum D, Low G, McEvoy S (2015) Silver metallization of titania particles: effects on photoactivity for the oxidation of organics. J Gastrointest Surg 19(8):1–6
Behnajady MA, Modirshahla N, Hamzavi R (2006) Kinetic study on photocatalytic degradation of C.I. acid yellow 23 by ZnO photocatalyst. J Hazard Mater 133(1-3):226–232
Leghari SAK, Sajjad S, Chen F, Zhang J (2011) WO3/TiO2 composite with morphology change via hydrothermal template-free route as an efficient visible light photocatalyst. Chem Eng J 166:906–915
Khot LR, Ehsani R, Sankaran S, Maja JM, Schuster EW (2012) Applications of nanomaterials in agricultural production and crop protection. A review, Crop Protection 35(C):64–70
Zheng L, Hong F, Lu S, Liu C (2005) Effect of nano-TiO2 on strength of naturally aged seeds and growth of spinach. Biol Trace Elem Res 104(1):83–91
Gl S, Gao Y, Wu H, Hou WH, Zhang CY, Ma HQ (2012) Physiological effect of anatase TiO2 nanoparticles on Lemna minor. Environ Toxicol Chem 31(9):2147–2152
Yang F, Liu C, Gao FQ, Su MY, Wu X, Zheng L, Hong FS, Yang P (2007) The improvement of spinach growth by nano-anatase TiO2 treatment is related to nitrogen photoreduction. Biol Trace Elem Res 119(1):77–88
Raliya R, Biswas P, Tarafdar JC (2015) TiO2 nanoparticle biosynthesis and its physiological effect on mung bean (Vigna radiata L). Biotechnology Reports 5:22–26
Yao KS, Wang DY, Ho WY, Yan JJ, Tzeng KC (2007) Photocatalytic bactericidal effect of TiO2 thin film on plant pathogens. Surface & Coatings Technology 201(15):6886–6888
Yao KS, Wang DY, Chang CY, Weng KW, Yang LY (2007) Photocatalytic disinfection of phytopathogenic bacteria by dye-sensitized TiO2 thin film activated by visible light. Surface & Coatings Technology 202(s 4–7):1329–1332
Cui HX, Yang GC, Jiang JF, Zhang P, Gu W (2013) Biological effects of PAS TiO2 sol on disease control and photosynthesis in cucumber (Cucumis sativus L.). Australian Journal of Crop Science 7(1):99–103
Vione D, Minero C, Maurino V, Carlotti ME, Picatonotto T, Pelizzetti E (2005) Degradation of phenol and benzoic acid in the presence of a TiO2-based heterogeneous photocatalyst. Applied Catalysis B: Environmental 58(1-2):79–88
Robert D (2003) Special issue on Industrial and Environmental Applications of Photocatalysis—parts 1 & 2 (Saint-Avold, France)—preface. Int J Photoenergy 5(2):43–44
Monnin T (2001) Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 293(5528):269–271
Malato S, Blanco J, Vidal A, Richter C (2002) Photocatalysis with solar energy at a pilot-plant scale: an overview. Appl Catal B: Environ 37(1):1–15
Pirkanniemi K, Sillanpää M (2002) Heterogeneous water phase catalysis as an environmental application: a review. Chemosphere 48(10):1047–1060
Bessekhouad Y, Robert D, Weber JV, Chaoui N (2004) Effect of alkaline-doped TiO2 on photocatalytic efficiency. J Photochem Photobiol A Chem 167(1):49–57
Photocatalysis HH, Pelizzetti E, Serpone N (1986) Homogeneous and heterogeneous photocatalysis. VNU Science Press, Maratea
Helz GR, Zepp RG, Crosby DG (1994) Aquatic and surface photochemistry. Lewis Publishers, BocaRaton
Sharma MVP, Kumari VD, Subrahmanyam M (2008) TiO2 supported over SBA-15: an efficient photocatalyst for the pesticide degradation using solar light. Chemosphere 73(9):1562–1569
Malato S, Blanco J, Caceres J, Fernandez-Alba AR, Aguera A, Rodriguez A (2002) Photocatalytic treatment of water-soluble pesticides by photo-Fenton and TiO2 using solar energy. Catal Today 76(2):209–220
Konstantinou IK, Albanis TA (2003) Photocatalytic transformation of pesticides in aqueous titanium dioxide suspensions using artificial and solar light: intermediates and degradation pathways. Appl Catal B-Environ 42(4):319–335
Daneshvar N, Aber S, Dorraji MSS, Khataee AR, Rasoulifard MH (2007) Photocatalytic degradation of the insecticide diazinon in the presence of prepared nanocrystalline ZnO powders under irradiation of UV-C light. Sep Purif Technol 58(1):91–98
Sharma MVP, Durgakumari VD, Subrahmanyam M (2008) Photocatalytic degradation of isoproturon herbicide over TiO2/Al-MCM-41 composite systems using solar light. Chemosphere 72(4):644–651
Adesina AA (2004) Industrial exploitation of photocatalysis: progress, perspectives and prospects. Catalysis Surveys from Asia 8(4):265–273
Brezová V, Blažková A, Karpinský L, Grošková J, Havl´ınová B, Jor´ık V, Èeppan M (1997) Phenol decomposition using Mn+/TiO2 photocatalysts supported by the sol-gel technique on glass fibres. J Photochem Photobiol A Chem 109(2):177–183
Zhang WY, Asiri AM, Liu Dl DD, Lin YH (2014) Nanomaterial-based biosensors for environmental and biological monitoring of organophosphorus pesticides and nerve agents. Trends Anal Chem 54(2):1–10
Hu XL, Li GS, Yu JC (2010) Design, fabrication and modification of nanostructured semiconductor materials for environmental and energy applications. Langmuir the Acs Journal of Surfaces & Colloids 26(5):3031–3039
Chen XB, Shen SH, Guo LJ, Mao SS (2010) Semiconductor-based photocatalytic hydrogen generation. Chem Rev 110(11):6503–6570
Liu SQ, Xie LJ, Zheng J, Jiang RF, Zhu F, Luan TG, Ouyang GF (2015) Mesoporous TiO2 nanoparticles for highly sensitive solid-phase microextraction of organochlorine pesticides. Analytica Chimica Acta 878:109–117.
Bao J, Hou CJ, Dong Q, Xiaoyu Ma XY, Chen J, Huo DQ, Yang M, Galil KHAE, Chen W, Lei Y (2016) ELP-OPH/BSA/TiO2 nanofibers/c-MWCNTs based biosensor for sensitive and selective determination of p-nitrophenyl substituted organophosphate pesticides in aqueous system. Biosens Bioelectro 85:935–942.
Awazu K, Fujimaki M, Rockstuhl C, Tominaga J, Murakami H, Ohki Y, Yoshidan N, Watanabe T (2008) A plasmonic photocatalyst consisting of silver nanoparticles embedded in titanium dioxide. J Am Chem Soc 130(5):1676–1680
Li HB, Li J, Xu Q, Yang ZJ, Hu XY (2013) A derivative photoelectrochemical sensing platform for 4-nitrophenolate contained organophosphates pesticide based on carboxylated perylene sensitized nano-TiO2. Anal Chim Acta 766(9):47–52
Li H, Li J, Yang Z, Xu Q, Hu X (2011) A novel photoelectrochemical sensor for the organophosphorus pesticide dichlofenthion based on nanometer-sized titania coupled with a screen-printed electrode. Anal Chem 83(13):5290–5295
Li H, Li J, Xu Q, Hu X (2011) Poly(3-hexylthiophene)/TiO2 nanoparticle-functionalized electrodes for visible light and low potential photoelectrochemical sensing of organophosphorus pesticide chlopyrifos. Anal Chem 83(24):9681–9686
Acknowledgements
This research was supported by the Beijing Municipal Natural Science Foundation (6164045), the Major National Scientific Research Program of China (2014CB932200), the National Key Research and Development Program of China (2016YFD0200500), the Basic Scientific Research Fund of National Nonprofit Institutes (BSRF201503), and the Agricultural Science and Technology Innovation Program.
Authors’ Contributions
YW collected and reviewed the data and drafted the manuscript. CS, XZ, and AW modified the first version of the draft and after revision. HC, ZZ, BC, and GL participated in discussions. YW and HC analyzed and interpreted the data. All authors read and approved the final manuscript.
Authors’ Information
YW is an associate professor. HC, ZZ, and GL are professors. CS, XZ, and BC are assistant professors, and AW is a graduate student in the Institute of Environment and Sustainable Development in Agriculture, Chinese Academy of Agricultural Sciences.
Competing Interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wang, Y., Sun, C., Zhao, X. et al. The Application of Nano-TiO2 Photo Semiconductors in Agriculture. Nanoscale Res Lett 11, 529 (2016). https://doi.org/10.1186/s11671-016-1721-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11671-016-1721-1