Abstract
The present study is objected to develop an analytical remote optical diagnostics of the functionalized carbons surface. Carbon composites with up to 1 mmol g−1 of irreversibly adsorbed bromine were produced by the room temperature plasma treatment of an activated carbon fabric (ACF) derived from polyacrylonitrile textile. The brominated ACF (BrACF) was studied by elastic optical scattering indicatrix analysis at wavelength 532 nm. The obtained data were interpreted within results of the thermogravimetric analysis, X-ray photoelectron spectroscopy and temperature programmed desorption mass spectrometry. The bromination dramatically reduces the microporosity producing practically non-porous material, while the incorporated into the micropores bromine induces the dielectric and structural impact on surface polarizability and conductivity due to the charging effect. We have found that the elastic optical scattering in proper solid angles in the forward and the backward hemispheres is sensitive to the kind of the bromine bonding, e.g., physical adsorption or chemisorption, and the bromination level, respectively, that can be utilized for the express remote fabrication control of the nanoscale carbons with given interfaces.
Similar content being viewed by others
Background
Nanoscale graphenes and related nanographite network systems have open edges, which adopt a nonbonding π-electron state [1–3]. This edge state exists at the degenerate point between the graphitic π and π* bands [4] and, definitely, it plays a role of the electron reservoir. As a result, the state population dynamic can effect on electronic/magnetic and optical, including nonlinear optical, properties [5–7]. A random disordered 3D structure of activated carbon fibers (ACFs) is frequently considered as a flexible network of the nanographite domains. Each domain originates from stacked graphene planes [3, 8] within interstitial nanosized voids—micropores [9]. The intercalation and guest-host interactions [2, 8, 9], including molecular adsorption, affect the properties of ACFs through the downshift of the Fermi level [2, 4, 5, 10]. Previously in [2], the bromination impact on the nanographite network magnetic properties was examined. Among the different kinds of nanographite, microporous ACFs provide an important model system with a variety of specific magnetic features manifestation related to its unique electronic structure [3, 10, 11]. The ACFs materials demonstrate an advanced response to the inclusion. The molecules and ions accommodated into the voids induce the charging effect, spin glass states [11–14], and can cause the magnetic switching [15, 16] and edge-state gas sensing [3, 8–12, 17]. So, the controlled bromination can be involved in the production of carbon interfaces for nanoscale sensing, charge/spin current modulation, nonvolatile magnetic switching, and optoelectronic applications [3, 11–13, 18–24]. The yield of bromine grafted over carbons by adsorption might be insufficient, while the radio frequency plasma bromination with the treatment time control is the reasonable route for the inclusion of the preset Br quantity. The plasma bromination gives much higher bromine content, up to 1.0 mmol g−1 of Br. In contrast to the high-temperature gas-phase halogenation [25], the high content of target surface functionalities could be reached even at the room temperatures. This extra quantity of the grafted bromine can be utilized for substitution and functionalization of various graphite carbons [18] with organic molecules to be implemented in nanoscale devices [21–24].
In order to monitor surface chemistry changes during the treatment process, express diagnostics methods should be elaborated for readout bromine guest-host interaction at the nanographite interface. The obtained data can be crucially important for the precise control of the carbons interfaces fabrication for specific applications. For this purpose, we applied elastic optical scattering indicatrix analysis. Comparison of optical scattering characterization with data of X-ray photoelectron spectroscopy (XPS) and temperature programmed desorption mass spectrometry (TPD-MS) techniques have shown the promising result for diagnostics application.
Methods
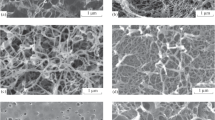
In this study, we examined a representative of the flexible microporous materials class – activated carbon fabric (ACF) that prepared from polyacrylonitrile textile by carbonization and steam activation. Nitrogen adsorption-desorption was measured on a TriStar Micromeritics C10900A porosimeter at −196 °C. The ACF has Brunauer-Emmett-Teller (BET) surface area S BET of ∼900 m2 g−1 and the total pore volume V tot of 0.19 cm3 g−1. According to scanning electron microscopy data-electron dispersion X-ray analysis (SEM-EDX) the ACF contains C = 92.7 mass% and O = 6.43–6.55 mass%. A cylindrical plasma treatment system for the radio frequency glow discharge fed with bromine (5ml min−1) was used for the surface functionalization. The (27.12 MHz) plasma treatment time was from 5 to 100 min at low pressure, ∼2 Pa. Hereafter, the brominated samples were assigned as BrACF “number”, where the “number” corresponds to the treatment duration in minutes. After the plasma treatment, SEM imagery on a Jeol JSM 7700 F microscope shows a practically flat surface, without new erosions and cracks that are observed at micrometer scale level (see Fig. 1).
Surface chemistry characteristics of the brominated ACF were studied by means of TPD-MS. Continuous scanning for the selected m/z ratio (positive mode) was done versus the temperature; at the residual pressure of ca. 10−4 Pa. TPD-MS data were registered, at a heating rate of 10 ° C min−1, according to the conditions described in [25, 26] and approved in [27]. XPS analysis was performed with a JPS-9030 photoelectron spectrometer at AlK α, 1486 eV.
Cross-sections of the elastic optical scattering indicatrix in forward and backward hemispheres were studied at the designed experimental platform (Fig. 2 a, b). A TEM00 beam of the CW DPSS laser at wavelength 532 nm, power P = 20 mW, was used as a light source. The samples were positioned perpendicularly to the incident beam and centered at the rotation axis of the setup. A diameter of the laser spot at the sample was about 3 mm that provides proper spatial averaging from the layers of the overlapped carbon fibers. Angular scattered light distribution measurement P(θ) was performed with a silicon photodiode PD (⌀ = 1 cm) placed on the rotation arm at the distance of 39 cm that corresponds to a solid angle ∼0.5 msr. The signal readout was performed by a 12-bit acquisition card with the sampling rate of 1000 samples per second. The setup acquisition dynamic range was extended up to eight orders of magnitude with neutral optical filter wheel FW insertion in front of the PD. A stepper motor with a self-locking worm gearbox provides full range (360°) rotation of the PD unit with an angular resolution of ∼ 0.05°. Overlapping of the PD unit with the incident laser beam during the angular scan produces a “blind” sector for the data acquisition that is presented as a hatched area in Fig. 4 a.
Results and Discussion
From the Volhard titration [25] and the EDX analysis of the BrACFs, the total bromine content, C Br, is within 0.11–0.99 mmol g −1 (see Additional file 1: Figure S1). It was shown that the treatment duration non-monotonously effects on C Br, it reaches the maximum for BrACF60. Prolongation of the treatment—from 60 to 100 min—reduces the C Br, due to the low-temperature discharge impact on the grafted bromine (Table 1).
From the BET adsorption measurements, the bromination significantly decreases the microporosity resulting in an almost non-porous material production. The S BET value reduces from 900 to 3–10 m2 g −1. The bromine accommodated into the micropores induces the dielectric and structural effects, including a polarizability of the interface layer modification [3]. In certain bromine substituents, p electrons are conjugated with π electrons in an aromatic ring [28, 29]. Moreover, a bromine substituent is a weak electron-withdrawing group so that π electrons would be delocalized [30]. The light absorption range of a compound containing a bromine substituent is broader than that for the initial one and should exhibit a redshift. Therefore, the ε value of the bromine-containing compound is higher than that of the corresponding reference compound. These variations can be readout by the elastic optical scattering analysis [31]. The SEM and N2 adsorption data testify that the bromination reaction takes place at the ACFs surface leading to sites formation enriched with grafted bromine—a kind of the nanoscale islands on the substrate interface. The sites are relatively hydrophobic due to the bromine terminal group. An interaction potential among the sites and its decay scale can be described in terms of the interaction of Br with the carbon interface states. This interaction is characterized by recording the Br 3d core level with an assistance of XPS.
According to the XPS data, the Br 3d peak shifts to a higher binding energy after ACF Br2 plasma treatment (see spectra 1 and 2 in Additional file 1: Figure S2). It indicates a Br 2 chemical state modification due to the bromine interaction with surface giving, being resulted in C n –Br2 nanoscaled islands coverage of the fibers interface. The reference data [32, 33] attributed Br 3d 5/2 peak at 67.4 ±0.2 eV to the strongly bonded Br in C n –Br2 interface complexes, while the peak at 69.2 ±0.2 eV – to the physisorbed Br or surface-bonded HBr. The Br 3d peak at 71.0 ±0.2 eV corresponds to the bromine covalent bonded to sp2 and sp3 hybridized C atoms [34–36]. Taking into account 1.05 eV splitting between 3d 5/2 and 3d 3/2, the Br 3d core level spectra can be attributed to the formation of all of the mentioned forms (see Additional file 1: Figure S2). The BrACF60 spectrum reflects the dominant contribution manifestation of the Br peak of the C–Br bond at ∼71.0 eV [36] with minor contributions of the contaminant species at 68.5 eV for Br − and Br2 at 67.4 eV [35]. The contaminant species contribution into the spectrum is negligible for the BrACF100. A tiny dispersion of the chemical shifts of the Br-species grafted on the surface significantly limits the application of XPS for determining an origin of the response.
Chemical derivatization was performed within the thermal excitation application. According to the TPD-MS data, the surface bromine desorbs from the ACFs surface via positive ionic fragments of m/z 79 and 81 (Br +) and 80 and 82 (HBr +). Typical temperature profiles of Br + and HBr + are presented in Fig. 3 a, b (see Additional file 1: Figure S3 for the rest ones). The profile data integration gives the following estimations of the integral intensity of HBr (I HBr) and Br (I Br) in the bromine-containing desorption products: 68.5–89.1 and 10.9–31.5% correspondingly. Consequently, the main gaseous product of surface grafted Br thermolysis is HBr. The molecular HBr desorbs at 250–700 °C.
The Br + profiles (Fig. 3 a, Additional file 1: Figure S3a-e) can be deconvoluted into a set of four components. Each component can be separated due to the pronounced difference of the desorption peak temperatures, see T (1−4) in Table 1. The desorption bands of different Br + species were registered in the temperature range of 145–195 °C (β 1(Br)), 228–317 °C (β 2(Br)), 309–425 °C (β 3(Br)), and 496–564 °C (β 4(Br)). These components can be attributed to the distinct surface centers adopting the bromine species. The centers have comparable bond strength with a certain type of bromine. They have also small differences of the thermal destruction and HBr desorption energies.
The HBr + profiles (Fig. 3 b, Additional file 1: Figure S3a*-e*) were deconvolved into three component set with HBr + desorption band of 304–385 °C (β 1(HBr)), 397–476 °C (β 2(HBr)), 541–583 °C (β 3(HBr)) that are shifted towards the ranges of the β # (Br) set. The profiles show a certain distribution of Br-bounded centers that differentiate by the desorption energy (Additional file 1: Figure S3a*-e*). So, it is difficult to draw a clear temperature boundary between β # (Br) components.
We attributed β 1(Br), β 2(Br), β 3(Br), and β 4(Br) components to the following bromine forms: (1) physisorbed, (2) physisorbed or intercalated in micropores, (3) chemisorbed on a more accessible surface, and (4) chemisorbed in tight micropores, correspondingly.
The less intense and fully symbate profiles of m/z 79 (Br +) and 80 (HBr +) at the temperature >300 °C can be explained by the HBr + dissociation in an ionic trap of the mass spectrometer. At the low-temperature mode < 300 °C the profiles of desorption of Br + and HBr + do not coincide due to the low-temperature desorption of physisorbed β 1(Br) form and a fraction of weakly-bonded bromine β 2(Br) forms.
A prolongation of the ACF plasma treatment induced (i) reducing the fraction of β 1(Br) and β 2(Br) with an increase of the contribution of β 3(Br) and β 4(Br) forms; (ii) the absolute content of β 1(Br), β 2(Br) and β 3(Br) in BrACFs passes through maxima at 15, 30, and 60 min of the treatment, respectively; (iii) the absolute content of the β 4(Br) substantially increases with the treatment time, (iv) a rise of the HBr fraction in the desorption products, see for details Additional file 1: Figure S3a*-e* and Tables 1 and 2. Based on the obtained results, the plasma bromination caused the gradual transformation of the physisorbed into the chemisorbed bromine that bound the most active centers of the ACF surface. The reductive properties of carbon surface and chemisorbed H (even presented in the form of adspecies) support the formation of HBr, as the major product of the surface bromine thermodesorption. The temperature increase over 300 °C promotes the breakage of the C–Br and C–H bounds. The free species are recombined into molecular HBr that desorbs under vacuum conditions.
Cross-sections of the BrACFs elastic optical scattering indicatrix under CW laser excitation at 532 nm are presented in Fig. 4 a. The obtained scattering signals were normalized on the total excitation power and smoothed. The relative error did not exceed 13% for each experimental curve. In general, it is shown that the scattered signal ε sc depends on the bromination time. Obtained polar plot can be separated into two groups of characteristic sectors (see Fig. 4 b) that correspond to the scattering in the forward (sectors A – |θ|<50° and B – 50°<|θ|<80°) and in the backward hemispheres (sectors C – 90°<|θ|<120° and D – 130°<|θ|<150°).
The scattering signal integrated into spherical coordinates [37, 38] for the sectors A, B, C, and D are presented in Fig. 5. The scattering in the forward hemisphere (sectors A and B) shows the monotonic growth with the bromine treatment time. In the case of scattering in the backward hemisphere (sectors C and D), the scattering magnitudes are higher and the signal demonstrates more complex dependence: an intensive growth of the signal for the short-time bromine treatment saturates with local maxima and turns to a slight monotonic growth.
In order to associate an obtained experimental data with chemical content, the simple regressive analysis can be used. Each integrated curve was linearly approximated versus the concentration of the different kinds of the bromine species. The most representative results of the proposed analysis are presented in Fig. 6. The sample BrACF100 was excluded from the analyzed datasets because it shows a more complicated scattering pattern. It requires more precise study for its response explanation.
In general, the obtained results show a correlation between HBr content and the elastic optical scattering in the backward hemisphere for the large scattering angles (see sector C in Fig. 6 a). For the smaller backscattering angles (sector D), the obtained experimental dependence ε sc(I HBr) is more complex. In sector C, the scattering signal shows a linear dependence ε sc∝I HBr with the factor of quality R 2=99%. In sector D, the dependence ε sc on I HBr is non-monotonous, but in the bromine treatment time range 10–30 min it has the same slope as the ε sc(I HBr) in sector C. The obtained non-monotonous character for sector D can be attributed to the scattered light redistribution in the vicinity of the exact backscattering condition (“blind” zone in Fig. 4 a). This phenomenon can be explained by the bromine incorporation into the micropores and the domination of the direct “metal” reflection from the composite surface. The last suggestion requires further research.
For the forward scattering, it was shown a linear correlation between the concentration of chemisorbed bromine Br (3+4) and the scattering at “smaller” (sector A) angle (see Fig. 6 b). For the large scattering angle (sector B), the level of the signals is lower and does not allow to attribute it to the certain kind of bromine contained in the BrACF.
The comparison of the light forward and the backscattering efficiencies ε sc in smaller angles (sectors A and D) versus the chemisorbed bromine concentration Br (3+4) is presented in Fig. 6 b. The level of the backscattering signal (sector D) is 2–3 times higher in comparison with the forward scattering one. We did not reveal distinct dependency of the ε sc in sector D on the Br (3+4) magnitude. It should be noted that the total bromine concentration is proportional to the chemisorbed bromine concentration, C Br∝Br(3+4). In sector A, the scattering signal shows a linear dependence ε sc∝Br(3+4) with the factor of quality R 2=89%. The obtained result is promising for the nondestructive express analysis of the chemisorbed bromine concentration in the synthesized BrACF samples.
Conclusions
In the present study, ACFs were brominated with a radio frequency vacuum technique. The surface functionalization was monitored in order to determine the optimal conditions for the BrACFs production. It can be used as precursors for the consequent applications. A different plasma treatment time was approved. The results indicated a possibility to functionalize ACFs with up to 1.0 mmol g −1 bromine within the single step production process. From SEM imagery, the plasma bromination has a minor effect on an anterior view of the individual fibers. XPS and TPD-MS studies reveal that the interaction between ACFs and the adsorbed bromine passes mainly via covalent bonding and physisorption interactions. The physisorbed bromine accommodated into the nanopores induces the dielectric and structural impact on the surface polarizability and conductivity due to the charging effect. The obtained results of the elastic optical scattering indicatrices analysis demonstrate (i) the correlation between the scattering in the forward hemisphere and chemisorbed bromine concentration and (ii) the correlation between the scattering in the backward hemisphere and the integral intensity of HBr + in TPD-MS profiles. The proposed optical diagnostics is a promising analytical technique for the surface characterization that can be adapted to related tasks. The scattering analysis can be used for ACFs surface characterization and measurements of the physisorbed and chemisorbed bromine concentration in the carbon materials.
Abbreviations
- ACF:
-
Activated carbon fabric
- BrACF:
-
Plasma brominated ACF
- SEM:
-
Scanning electron microscopy
- XPS:
-
X-ray
- Photoelectron spectroscopy; TPD-MS:
-
Thermoprogrammed desorption mass spectrometry
- EDX:
-
Electron dispersion X-ray
- S BET :
-
Brunauer-Emmett-Teller (BET) surface area
- V tot :
-
The total pore volume
References
Jang BZ, Zhamu A (2008) Processing of nanographene platelets (NGPs) and NGP nanocomposites: a review. J Mater Sci 43: 5092–5101. doi:10.1007/s10853-008-2755-2.
Yang Z, Gao R, Hu N, et al. (2012) The prospective two-dimensional graphene nanosheets: preparation, functionalization and applications. Nano-Micro Lett 4: 1–9. doi:10.1007/BF03353684.
Takai K, Kumagai H, Sato H, Enoki T (2006) Bromine-adsorption-induced change in the electronic and magnetic properties of nanographite network systems. Phys Rev B 73: 035435. doi:10.1103/PhysRevB.73.035435.
Harigaya K (2002) Theory on the mechanisms of novel magnetism in stacked nanographite. Mol Cryst Liq Cryst 386: 205–209. doi:10.1080/10587250215195.
Acik M, Chabal YJ (2011) Nature of graphene edges: a review. Jpn J Appl Phys 50: 070101. doi:10.1143/JJAP.50.070101.
Enoki T, Ando T (2014) Physics and chemistry of graphene: graphene to nanographene. CRC Press, Boca Raton.
Uklein AV, Diyuk VE, Grishchenko LM, Kozhanov VO, Boldyrieva OYu, Lisnyak VV, Multian VV, Gayvoronsky VYa (2016) Characterization of oxidized carbon materials with photoinduced absorption response. Appl. Phys. B 122: 287–296. doi:10.1007/s00340-016-6561-2.
Enoki T (2012) Nanographene-based host-guest systems. J Phys Chem Solids 72: 1408–1413. doi:10.1016/j.jpcs.2011.11.040.
Sato H (2002) Drastic effect of water-adsorption on the magnetism of nanomagnets. Solid State Commun 125: 641–645. doi:10.1016/S0038-1098(02)00880-3.
Enoki T, Suzuki M, Endo M (2003) Graphite intercalation compounds and applications. Oxford University Press, Oxford.
Enoki T, Kobayashia Y (2005) Magnetic nanographite: an approach to molecular magnetism. J Mater Chem 15: 3999–4002. doi:10.1039/b500274p.
Kheirabadi N, Shafiekhani A, Fathipour M (2014) Review on graphene spintronic, new land for discovery. Superlattices Microstruct 74: 123–145. doi:10.1016/j.spmi.2014.06.020.
Enoki T, Takai K (2008) Unconventional electronic and magnetic functions of nanographene-based host-guest systems. Dalton Trans 29: 3773–3781. doi:10.1039/b800138n.
Kiguchi M, Takai K, Joly JVL, Enoki T, Sumii R, Amemiya K (2011) Observation of magnetic edge state and dangling bond state on nanographene in activated carbon fibers. Phys Rev B Cond Matter 84: 045421. doi:10.1103/PhysRevB.84.045421.
Enoki T (2012) Role of edges in the electronic and magnetic structures of nanographene. Physica ScrT146: 014008. doi:10.1088/0031-8949/2012/T146/014008.
Enoki T (2012) Magnetism of nanographene. In: Rao CNR Sood AK (eds)Graphene: Synthesis, Properties, and Phenomena. Chapter 4.131–157.. Wiley-VCH, Weinheim. doi:10.1002/9783527651122.ch4.
Enoki T, Ando T (2014) Physics and chemistry of graphene: graphene to nanographene. CRC Press, Boca Raton.
Murakami M (2006) Dimensionality and applications of various graphite carbons. Rev High Press Sci Technol 16: 216–222. doi:10.4131/jshpreview.16.216.
Sattler K (2010) Handbook of nanophysics. CRC Press, Boca Raton.
Mansour AE, Dey S, Amassian A, Tanielian MH (2015) Bromination of graphene: a new route to making high performance transparent conducting electrodes with low optical losses. ACS Appl Mater Interfaces 7: 17692–17699. doi:10.1021/acsami.5b03274.
Kuila T, Bose S, Mishra AK, Khanra P, Kim NH, Lee JH (2012) Chemical functionalization of graphene and its applications. Prog Mater Sci 57: 1061–1105. doi:10.1016/j.pmatsci.2012.03.002.
Englert JM, Dotzer C, Yang G, et al (2011) Covalent bulk functionalization of graphene. Nature Chem 3: 279– 286. doi:10.1038/nchem.1010.
Georgakilas V, Otyepka M, Bourlinos AB, et al (2012) Functionalization of graphene: covalent and non-covalent approaches, derivatives and applications. Chem Rev 112: 6156–6214. doi:10.1021/cr3000412.
Friedrich JF, Wettmarshausen S, Hanelt S, Mach R, Mix R, Zeynalov EB, Meyer-Plath A (2010) Plasma-chemical bromination of graphitic materials and its use for subsequent functionalization and grafting of organic molecules. Carbon 48: 3884–3894. doi:10.1016/j.carbon.2010.06.054.
Diyuk VE, Zaderko AN, Veselovska KI, Lisnyak VV (2015) Functionalization of surface of carbon materials with bromine vapors at mediate high temperature: a thermogravimetric study. J Thermal Anal Calorim 120: 1665–1678. doi:10.1007/s10973-015-4495-2.
Gryn SV, Tsyrina VV, Kovalenko AS, Alekseev SA, Lisnyak VV, Ilyin VG (2009) Template-directed synthesis of dually porous periodic organosilicas with 1,5-bis-(2’-ethyl)-xylene bridging groups. Mater Chem Phys 114: 485–489. doi:10.1016/j.matchemphys.2008.09.067.
Diyuk VE, Mariychuk RT, Lisnyak VV (2016) Functionalization of activated carbon surface with sulfonated styrene as a facile route for solid acids preparation. Mater Chem Phys 184: 138–145. doi:10.1016/j.matchemphys.2016.09.034.
Cavallo G, Metrangolo P, Milani R, Pilati T, Priimagi A, Resnati G, Terraneo G (2016) The halogen bond. Chem Rev 116: 2478. doi:10.1021/acs.chemrev.5b00484.
Zhang C-Z, Li T, Yuan Y, Gu C-Y, Niu M-X, Cao H (2016) Effect of bromine substituent on optical properties of aryl compounds. J Phys Org Chem. doi:10.1002/poc.3620.
Zhang C-Z, Wang CY, Im C, Lu Y, Wang CS (2009) Significant effect of bromo substituents on nonlinear optical properties of polymer and chromophores. J Phys Chem B 114: 42–48. doi:10.1021/jp908073z.
Chu B (1974) Laser light scattering. Academic Press, New York.
Papirer E, Lacroix R, Donnett JB, Nanse G, Fioux P (1994) XPS study of the halogenation of carbon black – Part 1. Bromination. Carbon 32: 1341–1358. doi:10.1016/0008-6223(94)90121-X.
Vidic RD, Siler DP (2001) Vapor-phase elemental mercury adsorption by activated carbon impregnated with chloride and chelating agents. Carbon 39: 3–14. doi:10.1016/S0008-6223(00)00081-6.
Sasmaz E, Kirchofer A, Jew AD, Saha A, Abram D, Jaramillo TF, Wilcox J (2012) Mercury chemistry on brominated activated carbon. Fuel 99: 188–196. doi:10.1016/j.fuel.2012.04.036.
Zheng J, Liu H-T, Wu B, Di C-A, Guo Y-L, Wu T, Yu G, Liu Y-Q, Zhu D-B (2012) Production of graphite chloride and bromide using microwave sparks. Sci Rep 2: 662. doi:10.1038/srep00662.
Hanelt S, Friedrich JF, Orts-Gil G, Meyer-Plath A (2012) Study of Lewis acid catalyzed chemical bromination and bromoalkylation of multi-walled carbon nanotubes. Carbon 50: 1373–1385. doi:10.1016/j.carbon.2011.11.009.
Buchenko VV, Rodionova TV, Sutyagina AS, Goloborodko AA, Multian VV, Uklein AV, Gayvoronsky VYa (2016) Optical properties of thin nanosilicon films. Optic Mater 62: 612–620. doi:10.1016/j.optmat.2016.10.060.
Gayvoronsky VYa, Popov AS, Brodyn MS, Uklein AV, Multian VV, Shul’zhenko OO (2015) The effect of sintering temperature on linear and nonlinear optical properties of YAG nanoceramics In: Nanocomposites, Nanophotonics, Nanobiotechnology, and Applications, Vol. 156., 147–164.. Springer, Cham. doi:10.1007/978-3-319-06611-0_13.
Acknowledgements
VVL acknowledges a partial financial support from the National Scholarship Program of Slovak Republic for the Mobility of Students, Ph.D. Students, University Teachers, Researchers and Artists, SAIA Grant, no. in 2015. The authors would like to thank Dr. T.M. Bezugla for the source materials supplying and Dr. V.V. Trachevskyi from The National Aviation University in Kyiv for the research assistance. The authors VYaG, VVM, and FEK acknowledge a partial support from the NAS of Ukraine grant V-166.
Authors’ Contributions
VVL and VYaG supervised the project as scientific group leader and principal investigator. VVM and FEK performed the optical measurements and data analysis. AVV and LMG performed the brominated ACF purification and analysis. VED did TPD-MS data analysis and SEM and EDX investigations. VVL carried out XPS analysis. VED, VOK, OYuB, and VVL generalized physicochemical and analytical data. AVM conducted the TPD-MS studies. OYuB carried out the adsorption experiments. VED, VVL, and VYaG performed the general data analysis and discussion of the obtained data. All authors read and approved the final manuscript. VVL and VYaG wrote the manuscript. All authors read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional file
Additional file 1
Electronic Supplementary Material. (PDF 495 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Multian, V., Kinzerskyi, F., Vakaliuk, A. et al. Surface Response of Brominated Carbon Media on Laser and Thermal Excitation: Optical and Thermal Analysis Study. Nanoscale Res Lett 12, 146 (2017). https://doi.org/10.1186/s11671-017-1873-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11671-017-1873-7