Abstract
The universal problem of bacterial resistance to antibiotic reflects a serious threat for physicians to control infections. Evolution in bacteria results in the development of various complex resistance mechanisms to neutralize the bactericidal effect of antibiotics, like drug amelioration, target modification, membrane permeability reduction, and drug extrusion through efflux pumps. Efflux pumps acquire a wide range of substrate specificity and also the tremendous efficacy for drug molecule extrusion outside bacterial cells. Hindrance in the functioning of efflux pumps may rejuvenate the bactericidal effect of conventional antibiotics. Efflux pumps also play an important role in the exclusion or inclusion of quorum-sensing biomolecules responsible for biofilm formation in bacterial cells. This transit movement of quorum-sensing biomolecules inside or outside the bacterial cells may get interrupted by impeding the functioning of efflux pumps. Metallic nanoparticles represent a potential candidate to block efflux pumps of bacterial cells. The application of nanoparticles as efflux pump inhibitors will not only help to revive the bactericidal effect of conventional antibiotics but will also assist to reduce biofilm-forming capacity of microbes. This review focuses on a novel and fascinating application of metallic nanoparticles in synergy with conventional antibiotics for efflux pump inhibition.
Similar content being viewed by others
Review
The chronic infections identified with biofilms are difficult to eradicate as they are able to resist both antibiotics as well as the host immune system [1]. Biofilm barrier is one of the main reasons for conversion of acute to chronic infections [2]. As indicated by the report of the national institute of health and center of disease control, approximately 65–80% diseases are caused through biofilm-inducing bacteria, predominantly through Gram-negative bacteria Pseudomonas aeruginosa and Escherichia coli and Gram-positive bacterium Staphylococcus aureus [3]. Antibiotics have been appearing to be ineffective in treating infections having the biofilm, on account of their limited capacity to cross the biofilm rampart and to extirpate the targeted bacterial cells [4]. Additionally, bacteria have evolved a unique efflux system to drain out toxic substances and waste products outside the bacterial cell [5]. Efflux pumps are membrane-bound transporter proteins having a wide spectrum of substrate specificity and immense drug exclusion capacity [6].
All these diseases that associated concerns pertinent to biofilm and efflux pumps lead to the emergence of multidrug-resistant (MDR) bacteria or extensive drug-resistant (EDR) bacteria; owing to this, nanoparticles in conjunction with conventional antibiotics have been proposed as an alternative approach to eradicate or damage biofilm as well as to treat MDR or EDR infections.
These new antimicrobials, metallic nanoparticles, not only enhance the antimicrobial activity of existing antibiotics but also revive their bactericidal activity. The synergistic application of antibiotics and metallic nanoparticles exhibited more of their potential antimicrobial effect rather than their individual application [7, 8]. The utilization of nanoparticles with antibiotics as anti-biofilm or efflux pump inhibitor has been well examined and explored [1, 9,10,11]. Metallic nanoparticles have been extensively used to treat infections in human cell lines due to their low cytotoxicity (concentration dependent), high surface area, and broad-spectrum antibacterial activity [12,13,14]. Moreover, the combined application of metallic nanoparticles with an antibiotic reduces their concentration as drug dosage and hence diminishes the toxicity of both agents to human cell lines [15]. This review emphasizes the synergistic application of nanoparticles with antibiotics as anti-biofilm and efflux pump inhibitor on which extensive research has been carried out to combat infections caused by MDR or EDR pathogens.
Nanoparticles as Efflux Pump Inhibitors
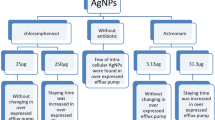
Various studies have been carried out to determine the mode of action of nanoparticles as a bactericidal agent. However, the several points on the mechanism of inhibitory action of nanoparticles on microorganisms still remain to be resolved. One of the possible mechanisms for bactericidal activity of nanoparticles is attributed to the inhibition of efflux pumps. Banoee et al. in 2010 adduce a novel efflux pump inhibitory role of zinc oxide nanoparticles on NorA efflux pumps of S. aureus. They have discovered 27 and 22% increase in the zone of inhibition for ciprofloxacin in the presence of zinc oxide nanoparticles in S. aureus and E. coli, respectively [16]. Afterward, Padwal et al. in 2014 propound the concept of synergistic use of polyacrylic acid-coated iron oxide (magnetite) nanoparticles (PAA-MNP) with rifampicin against Mycobacterium smegmatis with emphasis on the efflux inhibitory role of PAA-MNP. They have used a fusion of PAA-MNP and rifampicin in M. smegmatis which resulted in fourfold higher growth inhibition in contrast with rifampicin alone. It may be explained through threefold increased accumulation of antibiotic inside bacterial cells as proven with real-time transport studies on a common efflux pump substrate, ethidium bromide [17].
There are two possible mechanisms available through which metal nanoparticles can impede the working of efflux pumps. One possible mechanism is the direct binding of metal nanoparticles to the active site of efflux pumps, blocking the extrusion of antibiotics outside the cells. Metal nanoparticles may here act as a competitive inhibitor of antibiotic for the binding site of efflux pumps [18]. Another possible mechanism is through the disruption of efflux kinetics. The effect of silver nanoparticles for disruption of the efflux kinetics of MDR efflux pump, MexAM-OPrM, has already been examined in P. aeruginosa [19]. It may be suggested that metal nanoparticles may lead to termination of proton gradient followed by disruption of membrane potential or loss of proton motive force (PMF), resulting in deterioration of driving force essential for efflux pump activity [18, 20, 21]. However, the major constraint in the direct binding of nanoparticles with efflux pumps is their small size and reactivity. Additionally, nanoparticles may also bind with other membrane proteins rather than interacting simply with efflux pumps, and because of that, the chance of nanoparticles associating particularly with an efflux transporter each time amid the exposure is restricted.
Christena et al. has shown earlier in their studies regarding efflux inhibitory role of copper nanoparticles on the NorA efflux pump, partly due to the generation of Cu(II) ions from copper nanoparticles. This partial effect directly from copper nanoparticles may imply the direct interaction of nanoparticles with efflux pumps, supporting the first hypothesis, while partial effect due to release of Cu(II) ions might indicate the disruption of membrane potential and perturbing working of efflux pumps, supporting the second hypothesis [9]. Chatterjee et al. have also revealed the loss of membrane potential of E. coli cells from −185 to −105 and −75 mV after growing bacterial cells in the presence of 3.0 and 7.5 μg/ml concentration of copper nanoparticles, respectively, for 1 h [22]. The explicit mechanism for the efflux inhibitory role of nanoparticles still remains puzzling and requires further research.
Nanoparticles as Anti-Biofilm Agent
Biofilm provides resistance to bacteria, but this defiance gets intensified if biofilm is produced by drug-resistant bacteria [23]. Numerous studies have shown tremendous capabilities of metal nanoparticles to disintegrate thick biofilm barrier through the various modes of actions [24,25,26,27]. The penetrating power of metallic nanoparticles always remains a utile feature to employ them against biofilm infections [28,29,30]. This unique amalgamation of two diverse modalities, nanoparticles and antibiotic, paved a new way to combat against biofilm producing MDR or EDR bacteria.
One of the eloquent studies was conducted by Gurunathan et al. to elucidate the augmented bactericidal and anti-biofilm effect of different antibiotics with silver nanoparticles. Symbiotic use of ampicillin and silver nanoparticles greatly enhanced the biofilm inhibition in Gram-negative and Gram-positive bacteria by 70 and 55%, respectively, in contrast with approximately 20% biofilm inhibition after treated with silver nanoparticles alone. Similarly, the combined application of silver nanoparticles and vancomycin results in 55 and 75% biofilm inhibition in Gram-negative and Gram-positive bacteria, respectively [10]. These results suggest the alternative use of nanoparticle with antibiotics to induce biofilm inhibition opening clinical possibilities of novel therapy.
A similar effect was also observed for copper nanoparticles and zinc oxide nanoparticles using synergistically with antibiotics. According to this study, unification of copper nanoparticles and antibiotics showed more effective anti-biofilm activity in contrast with zinc oxide nanoparticle and antibiotic combination in both Gram-positive as well as Gram-negative bacteria. This increased inhibition with copper nanoparticles may be because of extrusion of Cu(II) ions generated from nanoparticles. Copper nanoparticles and zinc oxide nanoparticles coupled with specific antibiotic have exhibited enhanced anti-biofilm effect in the presence of 2% glucose, revealing increased bonding interactions between metallic nanoparticles and antibiotic in the presence of glucose [9]. Coating of metal nanoparticles with carbohydrates may transmogrify nanoparticle-cell interaction, cellular uptake, and cytotoxicity [31].
The Alliance Between Efflux Systems and Quorum Sensing
Efflux pumps play an important role in cell to cell signaling of biomolecules to assist biofilm formation. One probable mechanism to combat drug resistance is to employ the efflux pump inhibitors to block the quorum-sensing mechanism, ultimately hindering biofilm formation. Several studies have already been conducted to show the role of efflux pumps in quorum sensing [23, 32,33,34,35,36,37,38,39]. The explicit mechanistic relationship between efflux pumps and biofilm formation is still not fully understood. One viable justification could be the role of efflux pumps to extrude critical components required in quorum sensing. This functional aspect has already been proposed in earlier studies [40,41,42,43,44,45,46]. Impairment in the extrusion of signaling molecule through the application of efflux pump inhibitors may affect the process of quorum sensing or cell to cell signaling [47, 48] (Fig. 1).
Use of metal nanoparticles as an efflux pump inhibitor to impede extrusion of quorum-sensing signaling molecules (red filled circle) outside bacterial cells with the help of metal nanoparticles (yellow filled circle) to block efflux pump (filled cylinder) resulting in reduced binding of signaling molecule to its receptor (empty cylinder) and hindrance in biofilm formation
Another viable justification could be the role of efflux pumps to export toxic and waste by-product outside the cells. Rapidly metabolizing cells in the biofilm may rely upon the conductive system to extrude out the noxious and waste by-product resulting from various biochemical activities taking place inside bacterial cells [49]. This functional aspect has also been proposed in the study conducted by Kvist et al. in 2008 [50]. Employment of efflux pump inhibitors to block this conductive system may result in higher accretion of toxic by-product inside the bacterial cells, ultimately reducing biofilm formation (Fig. 2).
Use of metal nanoparticles as an efflux pump inhibitor to impede extrusion of toxic by-product of biochemical reactions (red filled circle) outside bacterial cells with the help of metal nanoparticles (yellow filled circle) to block efflux pump (filled cylinder) resulting in hindrance in biofilm formation
Some studies have also suggested the detriment of efflux pumps to affect cellular aggregation by transmuting cell membrane properties and ultimately affecting biofilm formation [47]. Numerous studies have already been conducted to deduce the effect of nanoparticles in combination with antibiotics as improved antibacterial and anti-biofilm agent. Table 1 contains a summary of these studies.
One of the recent studies has been conducted by Barapatre et al., deducing enhanced synergistic antibacterial and anti-biofilm activity of silver nanoparticles in combination with amikacin, kanamycin, oxytetracycline, and streptomycin antibiotic against Gram-positive and Gram-negative bacteria. Centralizing over green chemistry, silver nanoparticles were synthesized through the enzymatic reduction of silver nitrate by engaging two lignin-degrading fungus, viz., Aspergillus flavus and Emericella nidulans. It was suggested to use nanoparticles as a probe with conventional antibiotics to augment antibacterial and anti-biofilm activity against pathogenic microbes [51]. Disruption of ATP-dependent function like efflux pump inhibition has been reported as one of the potential mechanisms of synergistic effect of antibiotics and metallic nanoparticles [52].
A number of reports have successfully demonstrated appliance of nanoparticles against two diverse mechanisms of bacterial resistance, viz., MDR efflux pumps and biofilm formation, through which bacteria evade the action of conventional antibiotics. This review represents a new and promising approach to employ metallic nanoparticles in synergy with antibiotics as efflux pump inhibitor and anti-biofilm agent both to combat antibiotic resistance.
Conclusions
In the current scenario, there is an urge for an innovative approach for controlling MDR infections. Efflux pumps play dual roles, one is to extrude out antibiotics and the other is to assist in biofilm formation through expelling biomolecules important for quorum sensing, ultimately contributing to the virulence of bacterial pathogens. The blockage of MDR efflux pumps through nanoparticles will be helpful in both directions; it blocks the efflux of antibiotic outside the bacterial cells and hence increasing the effect of conventional antibiotics, and also, it blocks the efflux of quorum-sensing biomolecules and hence decreasing biofilm-forming capacity of bacterial cells. This approach reduces the need to conduct new research to investigate novel efflux pump inhibitor or new antibiotic but encourages the use of metal nanoparticles (employing as an efflux pump inhibitor) in synergy with conventional antibiotics. It will also assist in reducing the cost, the time, and the cytotoxicity problem of nanoparticles in the human cell lines which can endure a lower concentration of metallic nanoparticles. It would be a novel approach to target efflux pumps, reducing quorum-sensing signals in order to suppress biofilm formation.
Future Prospects
Bacterial evolution has resulted in the adoption of various mechanisms to revert the bactericidal effect of antibiotic and the host immune system. It leads to the generation of multidrug-resistant infections reflecting an urgent need to discover new approaches to fight against MDR or XDR infections. With the emergence of antibiotic resistance, symbiotic use of metallic nanoparticle with conventional antibiotics offers a better alternative for wrangling against antibiotic resistance. The application of nanoparticles as efflux pump inhibitors can be of great importance in two diversified directions but ending to one single outcome, i.e., to fight against bacterial infections. The exact mechanism of action of nanoparticles to block efflux pumps still needs to be investigated. It is likely that disruption of PMF could be a probable indirect mechanism by which nanoparticles might inhibit efflux. One of the major challenges with this approach is allied to the reactivity of nanoparticles that may render them to associate with other membrane proteins rather than efflux transporter proteins. This can be overcome by preparing targeted nanoparticles by linking them with anti-efflux monoclonal antibodies or lectins. This proper inhibition will calibrate them for focusing on a particular locale. Another considerable challenge may be the toxicity problem, using this approach owing to the large surface to volume ratio of small-sized nanoparticles which should be optimized before final validation.
References
Mu H, Tang J, Liu Q, Sun C, Wang T, Duan J (2015) Potent antibacterial nanoparticles against biofilm and intracellular bacteria. Scientific reports 6:18877–77
Halawani EM (2016) Nanomedicine opened new horizons for metal nanoparticles to treat multi-drug resistant organisms. Int J Curr Microbiol Appl Sci 5:397–414
Joo HS, Otto M (2012) Molecular basis of in vivo biofilm formation by bacterial pathogens. Chem & Biol 19:1503–13
LewisOscar F, MubarakAli D, Nithya C, Priyanka R, Gopinath V, Alharbi NS et al (2015) One pot synthesis and anti-biofilm potential of copper nanoparticles (CuNPs) against clinical strains of Pseudomonas aeruginosa. Biofouling 31:379–91
Sun J, Deng Z, Yan A (2014) Bacterial multidrug efflux pumps: mechanisms, physiology and pharmacological exploitations. Biochem and Biophys Res Commun 453:254–67
Nikaido H, Pagès JM (2012) Broad-specificity efflux pumps and their role in multidrug resistance of Gram-negative bacteria. FEMS Microbiol Rev 36:40–63
Li P, Li J, Wu C, Wu Q, Li J (2005) Synergistic antibacterial effects of β-lactam antibiotic combined with silver nanoparticles. Nanotechnology 16:1912
Fayaz AM, Balaji K, Girilal M, Yadav R, Kalaichelvan PT, Venketesan R (2010) Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: a study against gram-positive and gram-negative bacteria. Nanomedicine: Nanotechnology, Biology and Medicine 6:103–9
Ashajyothi C, Harish KH, Dubey N, Chandrakanth RK (2016) Antibiofilm activity of biogenic copper and zinc oxide nanoparticles-antimicrobials collegiate against multiple drug resistant bacteria: a nanoscale approach. J of Nanostruct in Chem 6:329–41
Gurunathan S, Han JW, Kwon DN, Kim JH (2014) Enhanced antibacterial and anti-biofilm activities of silver nanoparticles against Gram-negative and Gram-positive bacteria. Nanoscale Res Lett 9:373
Christena LR, Mangalagowri V, Pradheeba P, Ahmed KB, Shalini BI, Vidyalakshmi M et al (2015) Copper nanoparticles as an efflux pump inhibitor to tackle drug resistant bacteria. RSC Adv 5:12899–909
Sintubin L, Verstraete W, Boon N (2012) Biologically produced nanosilver: current state and future perspectives. Biotechnol and Bioeng 109:2422–36
Lewinski N, Colvin V, Drezek R (2008) Cytotoxicity of nanoparticles. Small 4:26–49
Kulshrestha S, Qayyum S, Khan AU (2017) Antibiofilm efficacy of green synthesized graphene oxide-silver nanocomposite using Lagerstroemia speciosa floral extract: a comparative study on inhibition of gram-positive and gram-negative biofilms. Microb Pathog 103:167–77
Kulshrestha S, Khan S, Hasan S, Khan ME, Misba L, Khan AU (2016) Calcium fluoride nanoparticles induced suppression of Streptococcus mutans biofilm: an in vitro and in vivo approach. Appl Microbiol Biotechnol 100:1901–14
Banoee M, Seif S, Nazari ZE et al (2010) ZnO nanoparticles enhanced antibacterial activity of ciprofloxacin against Staphylococcus aureus and Escherichia coli. J of Biomed Mater Res Part B: Appl Biomater 93:557–61
Padwal P, Bandyopadhyaya R, Mehra S (2015) Biocompatible citric acid‐coated iron oxide nanoparticles to enhance the activity of first‐line anti‐TB drugs in Mycobacterium smegmatis. J of Chem Technol and Biotechnol 90:1773–81
Padwal P, Bandyopadhyaya R, Mehra S (2014) Polyacrylic acid-coated iron oxide nanoparticles for targeting drug resistance in mycobacteria. Langmuir 30:15266–76
Nallathamby PD, Lee KJ, Desai T, Xu XH (2010) Study of the multidrug membrane transporter of single living Pseudomonas aeruginosa cells using size-dependent plasmonic nanoparticle optical probes. Biochemistry 49:5942–53
Choi O, Deng KK, Kim NJ, Ross L, Surampalli RY, Hu Z (2008) The inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growth. Water Res 42:3066–74
Dibrov P, Dzioba J, Gosink KK, Häse CC (2002) Chemiosmotic mechanism of antimicrobial activity of Ag+ in Vibrio cholerae. Antimicrob Agents and Chemother 46:2668–70
Chatterjee AK, Chakraborty R, Basu T (2014) Mechanism of antibacterial activity of copper nanoparticles. Nanotechnology 25:135101
Christena LR, Subramaniam S, Vidhyalakshmi M, Mahadevan V, Sivasubramanian A, Nagarajan S (2015) Dual role of pinostrobin—a flavonoid nutraceutical as an efflux pump inhibitor and antibiofilm agent to mitigate food borne pathogens. RSC Adv 5:61881–7
Morones-Ramirez JR, Winkler JA, Spina CS, Collins JJ (2013) Silver enhances antibiotic activity against gram-negative bacteria. Sci Transl Med 5:190ra81
Ansari MA, Khan HM, Khan AA, Cameotra SS, Saquib Q, Musarrat J (2014) Gum arabic capped‐silver nanoparticles inhibit biofilm formation by multi‐drug resistant strains of Pseudomonas aeruginosa. J of Basic Microbiol 54:688–99
Roe D, Karandikar B, Bonn-Savage N, Gibbins B, Roullet JB (2008) Antimicrobial surface functionalization of plastic catheters by silver nanoparticles. J of Antimicrob Chemother 61:869–76
Velázquez-Velázquez JL, Santos-Flores A, Araujo-Meléndez J, Sánchez-Sánchez R, Velasquillo C, González C et al (2015) Anti-biofilm and cytotoxicity activity of impregnated dressings with silver nanoparticles. Mater Sci and Eng: C 49:604–11
Denoncin K, Vertommen D, Paek E, Collet JF (2010) The protein-disulfide isomerase DsbC cooperates with SurA and DsbA in the assembly of the essential β-barrel protein LptD. J of Biological Chem 285:29425–33
Kadokura H, Katzen F, Beckwith J (2003) Protein disulfide bond formation in prokaryotes. Annu Rev of Biochem 72:111–35
Zheng M, Åslund F, Storz G (1998) Activation of the OxyR transcription factor by reversible disulfide bond formation. Science 279:1718–22
Kennedy DC, Orts-Gil G, Lai CH, Müller L, Haase A, Luch A, Seeberger PH (2014) Carbohydrate functionalization of silver nanoparticles modulates cytotoxicity and cellular uptake. J of nanobiotech 12:59
Baugh S, Phillips CR, Ekanayaka AS, Piddock LJ, Webber MA (2014) Inhibition of multidrug efflux as a strategy to prevent biofilm formation. J of Antimicrob Chemother 69:673–81
He X, Lu F, Yuan F, Jiang D, Zhao P, Zhu J et al (2015) Biofilm formation caused by clinical Acinetobacter baumannii isolates is associated with overexpression of the AdeFGH efflux pump. Antimicrob Agents and Chemother 59:4817–25
Yamasaki S, Wang LY, Hirata T, Hayashi-Nishino M, Nishino K (2015) Multidrug efflux pumps contribute to Escherichia coli biofilm maintenance. Int J of Antimicrob Agents 45:439–41
Hussain A, Ong EB, Phua KK, Balaram P, Ismail A (2014) Construction and characterization of a Salmonella enterica serovar Typhi tolC deletion mutant. Asian Pac J of Tropical Dis 4:226
Rosner JL, Martin RG (2013) Reduction of cellular stress by TolC-dependent efflux pumps in Escherichia coli indicated by BaeSR and CpxARP activation of spy in efflux mutants. J of Bacteriol 195:1042–50
Fiamegos YC, Kastritis PL, Exarchou V, Han H, Bonvin AM, Vervoort J et al (2011) Antimicrobial and efflux pump inhibitory activity of caffeoylquinic acids from Artemisia absinthium against gram-positive pathogenic bacteria. PLoS One 6, e18127
Matsumura K, Furukawa S, Ogihara H, Morinaga Y (2011) Roles of multidrug efflux pumps on the biofilm formation of Escherichia coli K-12. Biocontrol Sci 16:69–72
Liu Y, Yang L, Molin S (2010) Synergistic activities of an efflux pump inhibitor and iron chelators against Pseudomonas aeruginosa growth and biofilm formation. Antimicrob Agents and Chemother 54:3960–3
Evans K, Passador L, Srikumar R, Tsang E, Nezezon J, Poole K (1998) Influence of the MexAB-OprM multidrug efflux system on quorum sensing in Pseudomonas aeruginosa. J of Bacteriol 180:5443–7
Pearson JP, Van Delden C, Iglewski BH (1999) Active efflux and diffusion are involved in transport of Pseudomonas aeruginosa cell-to-cell signals. J of Bacterial 181:1203–10
Chan YY, Chua KL (2005) The Burkholderia pseudomallei BpeAB-OprB efflux pump: expression and impact on quorum sensing and virulence. J of Bacteriol 187:4707–19
Chan YY, Bian HS, Tan TM, Mattmann ME, Geske GD, Igarashi J et al (2007) Control of quorum sensing by a Burkholderia pseudomallei multidrug efflux pump. J of Bacteriol 189:4320–4
Pumbwe L, Skilbeck CA, Wexler HM (2008) Presence of quorum-sensing systems associated with multidrug resistance and biofilm formation in Bacteroides fragilis. Microb ecology 56:412–9
Yang S, Lopez CR, Zechiedrich EL (2006) Quorum sensing and multidrug transporters in Escherichia coli. Proc of the Natl Acad of Sci of the United States of Am 103:2386–91
Martinez JL, Sánchez MB, Martínez-Solano L, Hernandez A, Garmendia L, Fajardo A et al (2009) Functional role of bacterial multidrug efflux pumps in microbial natural ecosystems. FEMS Microbiol Rev 33:430–49
Nigam A, Gupta D, Sharma A (2014) Treatment of infectious disease: beyond antibiotics. Microbiological Res 169:643–51
Varga ZG, Armada A, Cerca P, Amaral L, SUBKI MA, Savka MA et al (2012) Inhibition of quorum sensing and efflux pump system by trifluoromethyl ketone proton pump inhibitors. In Vivo 26:277–85
Fahmy A, Srinivasan A, Webber MA (2016) The relationship between bacterial multidrug efflux pumps and biofilm formation. In: Li XZ, Elkins CA, Zgurskaya HI (eds) Efflux-Mediated Antimicrobial Resistance in Bacteria. Springer International Publishing., pp 651–63
Kvist M, Hancock V, Klemm P (2008) Inactivation of efflux pumps abolishes bacterial biofilm formation. Appl and Environ Microbiol 74:7376–82
Barapatre A, Aadil KR, Jha H (2016) Synergistic antibacterial and antibiofilm activity of silver nanoparticles biosynthesized by lignin-degrading fungus. Bioresour and Bioprocess 3:8
Hwang IS, Hwang JH, Choi H, Kim KJ, Lee DG (2012) Synergistic effects between silver nanoparticles and antibiotics and the mechanisms involved. J of Med Microbiol 61:1719–26
Acknowledgements
This work was supported by (Promotion of University Research and Scientific Excellence) DST-PURSE Program Phase-II No. SR/PURSE Phase 2/9.
Funding
This study is supported by the internal funding of the department.
Author information
Authors and Affiliations
Contributions
DG wrote first draft; AS helped in the writing as well as critical proof-reading; and AUK conceived the idea and gave the direction and checked the review. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Gupta, D., Singh, A. & Khan, A.U. Nanoparticles as Efflux Pump and Biofilm Inhibitor to Rejuvenate Bactericidal Effect of Conventional Antibiotics. Nanoscale Res Lett 12, 454 (2017). https://doi.org/10.1186/s11671-017-2222-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11671-017-2222-6