Abstract
A ternary composite of poly (3,4-ethylenedioxythiophene)/gold/graphene (PEDOT/Au/GO) for promising electrochemical sensor was synthesized by solid-state heating method. The interaction between the PEDOT, Au, and GO explored for detection of nitrite and iodate. It was found that the PEDOT/Au/GO composite had shale-like morphology with a uniform distribution of gold nanoparticles. Electrochemical experiments showed that the PEDOT/Au/GO composite modified electrode exhibited good electrocatalytic activity toward determination of iodate. The amperometric experiments at the PEDOT/Au/GO/GCE revealed that a good linear relationship existed between peak current and the concentration in the range of 100–1000 μM with the detection of 0.53 and 0.62 μM (S/N = 3) for nitrite and iodate, respectively. Moreover, the current response of PEDOT/Au/GO/GCE for nitrite and iodate at 10 μM was up to 9.59 and 11.47 μA, respectively.

Mechanisms of the direct electron transfer between ion(nitrite or iodate)and the PEDOT/Au/GO composite
Similar content being viewed by others
Background
Nitrite (NO2 −) is ubiquitous within environmental, food, and agricultural products, which was recognized to exist in physiological systems when ingest compounds contain NO2 − [1, 2]. NO2 − can react with amines to form carcinogenic nitrosamines, and the continuous ingestion of these ions can be harmful to animal and human health [3,4,5]. Also with the other ion press close to our daily life, iodate (IO3 −), the iodized salt, is recognized as the most successful strategy for the prevention of iodide deficiency disorders. However, an excess of IO3 − can produce goiter and hypothyroidism as well as hyperthyroidism [6, 7]. Therefore, many techniques have been developed for NO2 − and IO3 − detection [8], including spectroscopic [9], chromatographic [10], chemiluminescence [11], electrochemical [12,13,14,15], and capillary electrophoresis methods [16]. Among them, the electrochemical method has been widely used due to its high sensitivity, simplicity, rapidness, and low cost. Generally, the electrodes have modified with nanostructured metal (such as Pt, Au), metal oxide (such as WO3, RuO2), and carbon nanomaterials, and which have been extensively investigated for the development of effective electrochemical sensors [17,18,19,20]. Among them, Au nanoparticles have broad applications in the field of electrochemical sensors with its ideal catalytic activity, sensitivity, biocompatibility, interface-dominated properties, excellent conductivity, and high signal to noise ratio. However, high cost, poor selectivity, and instability of Au make it unsuitable for practical applications [21].
Recently, the conducting polymer/gold hybrid materials have been extensively investigated to obtain new kind composite materials with synergetic or complementary behaviors [22, 23]. As one of the typical and important part of conducting polymers, poly (3,4-ethylenedioxythiophene) (PEDOT) has wide applications in the field of displays, smart windows, sensors, capacitors, batteries, and photovoltaic devices [24,25,26]. Generally, in chemically synthesized PEDOT/Au composites, electrocatalytic performances of the composite could be enhanced through Au–S(thiophene) interactions and the activation of metal ion coordination [27, 28]. And many reports have been published for the preparation of binary PEDOT/Au composites [29, 30].
In recent years, most of the researches focus on the preparation of graphene/conducting polymer-based ternary composites because of graphene-based carbon materials have high surface area, unique electronic transport property, high electrocatalytic activity, and good chemical stability [31, 32]. These unique characteristics of graphene-based carbon materials possibly bring unique chemical structures and more superior performance to the composites [33].
Yao et al. synthesized a PANI/MWNTs/Au composite sensor for detection of NO2 −, and the current response was about 2.8 μA for 10 μM NO2 − [34]. Xue et al. prepared a ternary nanocomposite of gold nanoparticles/polypyrrole/graphene by facile wet-chemical routes and found that as-prepared composites have a good electrocatalytic activity toward the glucose with its high sensitivity [35]. In this case, research on the preparation, structure, and properties of graphene-based ternary nanocomposites will be very interesting and challenging in the fields of sensors. However, the conventional chemical and electrochemical technique for ternary nanocomposite is usually complicated and tedious. Therefore, cost-effective, clear, green, simple, and high-efficiency synthetic methods are desirable.
Herein, we report the fabrication of a ternary composite (PEDOT/Au/GO) of poly (3,4-ethylenedioxythiophene), gold nanoparticles, and graphene for promising electrochemical sensor by solid-state heating method. For comparison, the pure PEDOT and binary composite (PEDOT/Au) were also synthesized in the similar manner. The PEDOT/Au/GO and PEDOT/Au composite have been used for the electrochemical sensitive determination of iodate. And the PEDOT/Au/GO composite was selected for evaluating its potential application as electrochemical sensor for detection of nitrite and iodate on the basis of systematic studies on the amperometric determination of nitrite and iodate.
Experimental
Chemicals and Reagents
3,4-Ethylenedioxythiophene (EDOT) was obtained from Shanghai Aladdin Reagent Company (China), and it was purified by distillation under reduced pressure and stored in a refrigerator prior to use. Chloroauric acid hydrated (HAuCl4·4H2O) was purchased from Shanghai Aladdin Reagent Company (China). Graphene (GO) was purchased from Strem Chemicals Inc. (USA). All other reagents were of analytical grade and used as supplied without further purification. 2,5-Dibromo-3,4-ethylenedioxythiophene was synthesized according to the previous report [36].
Synthesis of the PEDOT/Au/GO and PEDOT/Au composites
Before the synthesis of composites, the Au nanoparticle sol solution was prepared in advance. The Au nanoparticle sol solution was prepared by reducing HAuCl4 with NaBH4 as reductant. A typical preparation of Au nanoparticle sol solution was as follows: 60 mg of HAuCl4·3H2O was added to 100 mL of water to create HAuCl4 solution. A total of 3.4 mL of aqueous solution of Na3C6H5O7 (1%) was then added to the 40 mL of HAuCl4 solution under vigorous stirring for 10 min. The 1.2 mg NaBH4 was then quickly added, and the color of the solution immediately turned into a purple.
A typical solid-state heating synthesis of PEDOT/Au/GO composite was as follows (Fig. 1): a mixture of 0.5 g (2 mmol) monomer (2,5-dibromo-3,4-thylene dioxythiophene) and 10 mg GO in 30 mL chloroform were ultrasonicated for 30 min to facilitate monomer to adsorb on the surface of GO. The mixture was then allowed to evaporate the chloroform. The residue was put in a mortar followed by constant grinding for 5 min. Then the mixture was added to the Au nanoparticle sol solution and stirred for 10 min. The mixture was then filtered and washed by distilled water, at last kept in a vacuum oven at 60 °C for 24 h. The obtained product was denoted as PEDOT/Au/GO composite.
For comparison, the binary composite (PEDOT/Au) and pure PEDOT were also synthesized in the similar manner.
Structure Characterization
Fourier-transform infrared (FTIR) spectra of the samples were recorded on a BRUKER-QEUINOX-55 FTIR spectrometer using KBr pellets. UV-vis spectra of the samples were recorded on a UV-visible spectrophotometer (UV4802, Unico, USA). The samples for TEM measurements were prepared by placing a few drops of products ethanol suspension on copper supports and performed on a Hitachi 2600 electron microscope. The elemental content of the sample was characterized by energy-dispersive X-ray spectroscopy (EDS), which was taken on a Leo1430VP microscope with operating voltage 5 kV. EDX experiments were carried out with a pellet which was pressed at 200 MPa and then adhered to copper platens.
Measurement of Electrocatalytic Activity
Cyclic voltammetry (CV) and amperometric i–t curve were performed on electrochemical workstation CHI 660C (ChenHua Instruments Co., Shanghai, China). Three-electrode system was employed to study the electrochemical performance of composite. Pt electrode was used as a counter electrode and saturated calomel electrode (SCE) as a reference electrode. PEDOT/Au/GO composite modified GCE (glassy carbon electrode; diameter = 3 mm) was used as a working electrode. The working electrode was fabricated by placing 5 μL of 30 mg/L PEDOT/Au/GO composite suspension (The PEDOT/Au/GO composite was dispersed in water to create suspension (30 mg/L).) on a bare GCE surface and air dried for 10 min. All the experiments were carried out at ambient temperature and air atmosphere.
Results and Discussion
Figure 2a represents the FTIR spectra of PEDOT, PEDOT/Au, and PEDOT/Au/GO. As can be seen in Fig. 2a, the spectrum of PEDOT/Au/GO and PEDOT/Au composites are similar to that of pure PEDOT, indicating a successful formation of polymer in composite. The two bands appearing at ~ 1514 and ~ 1324 cm−1 are assigned to the asymmetric stretching mode of C=C and inter-ring stretching mode of C–C, respectively. The bands appearing at ~ 1198, ~ 1140, and ~ 1084 cm−1 are attributed to the C–O–C bending vibration in ethylenedioxy. These results are in good agreement with the previous reported FTIR spectra of PEDOT [37]. Although the spectra of PEDOT/Au/GO and PEDOT/Au composites are similar to that of pure PEDOT, several discrepancies occur between pure PEDOT and composites. According to the previous report, the polymerization degree of polythiophene can be evaluated from the ratio of integration of the infrared bands at 690 and 830 cm−1 [38, 39], and the higher degree of polymerization can be resulted from relatively lower value of that intensity ratio. Therefore, it can be deduced from Fig. 2a that the polymerization degree of the PEDOT/Au/GO, PEDOT/Au, and PEDOT is in the order of PEDOT/Au/GO > PEDOT/Au > PEDOT, which suggests that the PEDOT/Au/GO has a higher polymerization degree than PEDOT/Au and PEDOT. Furthermore, this result indicates that the presence of GO in reaction medium can play positive role in increasing the polymerization degree of PEDOT in composite matrix.
Figure 2b shows the UV-vis absorption spectra of PEDOT, PEDOT/Au, and PEDOT/Au/GO. As shown in Fig. 2b, the PEDOT displays broad absorption peak starting at ~ 500 nm and extending into the near-infrared region. This absorption feature, known as a “free carrier tail,” correlates with the conductivity of the polymers. The presence of this absorption peak has been shown to correspond to the polymer having a longer conjugation length and greater order, which allows for greater mobility of charge carriers [40, 41]. In the case of composites, the PEDOT/Au exhibits a similar absorption feature to that of PEDOT, while PEDOT/Au/GO displays a absorption peak (π-π* transition) at ~ 500 nm along with a free-carrier tail extending into the near-infrared region [37, 40, 42]. This phenomenon further implies that there is strong interaction between aromatic regions of the non-covalent graphene and quinoid rings of PEDOT [43, 44].
Figure 3 shows the transmission electron micrograph (TEM) images of PEDOT, PEDOT/Au, and PEDOT/Au/GO. As depicted in Fig. 3a, b, pure PEDOT exhibits shale-like morphology with layered structure, while the PEDOT/Au composite had granular-like morphology mixing from PEDOT and Au nanoparticles with an average size of 50 nm. However, in the case of PEDOT/Au/GO composite (Fig. 3c), it is found that the composite had shale-like morphology with a uniform distribution of gold nanoparticles (dark-shaded nanoparticles). Furthermore, the shale-like morphology of PEDOT/Au/GO composite is constructed from light-shaded and dark-shaded layered structure, which can be attributed to the GO and PEDOT, respectively. These results imply that the GO and Au nanoparticles are not simply mixed up or blended with the PEDOT, suggesting that the GO and Au nanoparticles (average size of 10~15 nm) are embedded in composite matrix. This uniform distribution of GO and Au nanoparticles in composite may be related to the shale-like morphology of PEDOT, which can bring some possibility for formation of lamellar structures from incorporation of PEDOT and GO, and leads a large surface area for uniform distribution Au nanoparticles.
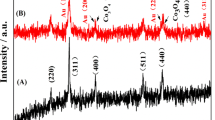
Figure 4a indicates the XRD patterns of PEDOT, PEDOT/Au, and PEDOT/Au/ GO. In addition, to study the element percentage of Au, energy-dispersive X-ray (EDX) spectroscopies of PEDOT, PEDOT/Au, and PEDOT/Au/GO are also shown in Fig. 4b. As depicted in Fig. 4a, the PEDOT, PEDOT/Au, and PEDOT/Au/GO display broad diffraction peaks with low intensity at 2θ~25.9°, which can be associated to the intermolecular spacing of polymer backbone or assigned to the (020) reflection [45]. In addition, the composite shows sharp diffraction peak at 2θ~26°, indicating the existence of GO in composite [46]. In the case of PEDOT/Au/GO composite, the characteristic diffraction peak of PEDOT (2θ~25.9°) is overlapped with that of GO (2θ~26.6°). The XRD pattern of composite indicates that the presence of characteristic diffraction peaks of Au (four peaks with low intensity at 2θ values of 37.9° and 43.7°), which correspond to Bragg’s reflections from the (111) and (200) planes of Au [47], suggesting the successful incorporation of Au in the composite, which is in accordance with the result of EDX (Fig. 4b) of PEDOT/Au (presence of 1.92 wt% Au). However, there is no obvious diffraction peak for Au in PEDOT/Au/GO, which is not matched with the result of EDX (Fig. 4b) of PEDOT/Au/GO (presence of 1.71 wt% Au). This may be attributed to the small particle size and high dispersion of Au nanoparticles in PEDOT/Au/GO composite, and this phenomenon is similar to the observation in Au/Zn nanocomposite, which did not show any diffraction peak for Au nanoparticles [47].
Thermogravimetric analysis of PEDOT, PEDOT/Au, and PEDOT/Au/GO are shown in Fig. 5. It is clear that these samples undergo three-step weight loss behaviors. The first step weight loss at 40–104 °C is due to the loss of trace of trapped water or moisture from the polymer chain. The second step weight losses occur at 112 to 323 °C with the weight loss of 24.78% (PEDOT), 24.33% (PEDOT/Au), and 19.17% (PEDOT/Au/GO), respectively. This is due to the loss of low molecular weight polymer. In the third step, polymer undergoes degradation after 323 °C. This result signpost that polymer is stable up to 323 °C. And present residual weight percentages of 20.8% (PEDOT), 29.1% (PEDOT/Au), and 36.5% (PEDOT/Au/GO) after 800 °C. These results suggest that presence of the Au and GO can enhance the thermo stabilities of the composites.
To evaluate the potential application of PEDOT/Au/GO and PEDOT/Au composites as electrochemical sensor, the iodate (IO3 −) is selected as testing specie for electrochemical experiment. Figure 6 shows cyclic voltammograms of PEDOT/Au/GO and PEDOT/Au composites in 0.1 M H2SO4 solution containing 5 mM iodate. As shown in Fig. 6, there is no oxidation/reduction peak in the both case of PEDOT/Au/GO (PEDOT/Au/GO/GCE) and PEDOT/Au modified glass carbon electrode (PEDOT/Au/GCE) without adding IO3 −. When the IO3 − is added, both composites display a couple of oxidation/reduction peaks, and the reduction peak current value is higher than that of respective oxidation peak, which is resulted from the reduction of IO3 − to I− [48]. Furthermore, the highest reduction current intensity occurs in the case of PEDOT/Au/GO/GCE, suggesting that PEDOT/Au/GO/GCE has an enhanced electrochemical catalytic activity than PEDOT/Au/GO.
Figure 7 shows cyclic voltammograms of PEDOT/Au/GO/GCE in 0.025 M PBS (pH = 6.86) solution containing nitrite (Fig. 7a and 0.1 M H2SO4 solution containing iodate (Fig. 7b), respectively. The peak current increases with the increase of nitrite concentration (3 to 15 mM) and iodate concentration (2 to 20 mM), respectively. As seen in Fig. 7a, there is broad oxidation peak at about 0.82 V, which can be assigned to the conversion of NO2 − to NO3 − through a two-electron oxidation process [49]. In the case of the iodate (Fig. 7b), the reduction peak currents increases and the peak potential slightly shifts from 300 to 160 mV, which can be attributed to the rapid reduction of IO3 − to I− [48].
Figure 8 shows the steady-state catalytic current-time response of PEDOT/Au/GO/GCE with successive addition of 1.0 × 10−5, 1.0 × 10−4, and 1.0 × 10−3 M nitrite (Fig. 8, potential controlled at 0.78 V) and iodate (Fig. 8b, potential controlled at − 0.25 V), respectively. As shown in Fig. 8, a well-defined response is observed under the successive addition of 1.0 × 10−5, 1.0 × 10−4, and 1.0 × 10−3 M nitrite and iodate, respectively.
Figure 9 shows the steady-state catalytic current-time response of PEDOT/Au/GO/GCE with successive addition of 1.0 × 10−3 M nitrite (Fig. 9a, potential controlled at 0.78 V) and iodate (Fig. 9b, potential controlled at − 0.25 V). The results from Fig. 9 show that the detection of both nitrite and iodate has better steady-state catalytic current in the range of 100–1000 μM, and the response time is about 4 s after each addition of nitrite and iodate, respectively. The plots of chronoamperometric currents vs. ion concentration (insets in Fig. 9) further indicate a good linear relationship exists between peak current and the concentration in the range of 100–1000 μM with the linear equations of I (μA) = 0.0322 C + 26.422 (R 2 = 0.9995) and I (μA) = 0.13757C + 6.80312 (R 2 = 0.999) for nitrite and iodate, respectively. Most importantly, the detection of nitrite and iodate by PEDOT/Au/GO/GCE exhibits a step response and has an ideal current response for electrochemical detection for nitrite and iodate with loading of small amount of composite (5 μL from 30 mg/L) on glassy carbon electrode. Besides, the low detection limit are estimated to be 0.53 μM and 0.62 μM (S/N = 3) for nitrite and iodate, respectively.
The comparisons for the parameters of nitrite and iodate detection by various chemically modified electrodes are listed in Table 1. The comparison results show that the response of PEDOT/Au/GO/GCE modified electrode has a lower current (9.59 μA) than that (17.5 μA) of MWNT-PAMAM-Chit in addition of 10 μM nitrite. However, the current response of PEDOT/Au/GO/GCE for addition of 10 μM nitrite is higher than that (0.3 μA) of Nano-Au/P3MT/GCE. In addition, the current response of PEDOT/Au/GO composite is 11.47 μA for addition of 10 μM iodate, which also gives a better proof that the PEDOT/Au/GO/GCE-modified electrode is suitable [25] for the detection for iodate.
Figure 10 shows the PEDOT/Au/GO/GCE composite modified electrode imparts higher stability onto amperometric measurements of analyte (1.0 mM nitrite or 1.0 mM iodate) during prolonged 1000 s experiment. The response remains stable throughout the experiment, indicating no inhibition effect of iodate and its reduction products for modified electrode surface. However, comparing with iodate, the response remains unstable in the case of nitrite.
Figure 11 shows the mechanisms of the direct electron transfer between ion (nitrite or iodate) and GCE (glassy carbon electrode) through the PEDOT/Au/GO/GCE composite. As depicted in Fig. 11, the shale-like PEDOT can incorporate with GO to form a lamellar structure, which can lead a large surface area for uniform distribution of Au nanoparticles. Furthermore, the generated electrons will conduct to GCE via the shortest resistance path through highly conductive GO dispersed in composite as illustrated in Fig. 11. However, without GO, the electrons will have to go through PEDOT medium, which has considerable resistance that causes significant potential drop and much lower electron transfer rate. Therefore, GO plays an important role in facilitating the electron exchange between ion (nitrite or iodate) and GCE because it forms conductive matrix leading to reduced electrical resistance paths.
Real Sample Analysis
In order to validate/test the practical application of the modified electrode, the PEDOT/Au/GO/GCE was applied for detection of nitrite concentration in tap water with standard addition method. A certain volume of samples was added to electrochemical cell for the determination of nitrite by amperometric determination. As shown in Table 2, the recovery of the sample ranged from 98.4 to 104.3%. Therefore, the PEDOT/Au/GO/GCE could be used for the detection of nitrite in water sample.
Conclusion
A ternary composite of PEDOT/Au/GO for promising electrochemical sensor was synthesized by solid-state heating method. The results revealed that the shale-like morphology of PEDOT might bring some possibility for formation of lamellar structures from incorporation of PEDOT in GO matrix, which could lead a large surface area for uniform distribution of Au nanoparticles. Therefore, the synergistic effect between PEDOT, GO, and Au nanoparticles as well as the large contact surface area of composite led the PEDOT/Au/GO composite display a strong electrocatalytic activity toward the oxidation of nitrite and reduction of iodate. And the current responses of the detection of nitrite and iodate were high enough to achieve an obvious step response. Furthermore, the PEDOT/Au/GO composite had an ideal current response for electrochemical detection for nitrite and iodate with loading of small amount of composite (5 μL from 30 mg/L) on glassy carbon electrode.
References
Bertotti M, Pletcher D (1997) Amperometric determination of nitrite via reaction with iodide using microelectrodes. Anal Chim Acta 337:49–55
Chen S-S, Shi Y-C, Wang A-J, Lin X-X, Feng J-J (2017) Free-standing Pt nanowire networks with clean surfaces: highly sensitive electrochemical detection of nitrite. J Electroanal Chem 791:131–137
Chow C, Hong C (2002) Dietary vitamin E and selenium and toxicity of nitrite and nitrate. Toxicology 180:195–207
Shi Y-C, Feng J-J, Chen S-S, Tu G-M, Chen J-R, Wang A-J (2017) Simple synthesis of hierarchical AuPt alloy nanochains for construction of highly sensitive hydrazine and nitrite sensors. Mater Sci Eng: C. 75:1317–1325
Huang SS, Liu L, Mei LP, Zhou JY, Guo FY, Wang AJ, Feng JJ (2016) Electrochemical sensor for nitrite using a glassy carbon electrode modified with gold-copper nanochain networks. Microchim Acta 183:791–797
Chatraei F, Zare HR (2013) Nano-scale islands of ruthenium oxide as an electrochemical sensor for iodate and periodate determination. Mat Sci Eng C -Mater 33:721–726
Li R, Xu P, Fan J, Di J, Tu Y, Yan J (2014) Sensitive iodate sensor based on fluorescence quenching of gold nanocluster. Anal Chim Acta 827:80–85
Tominaga M, Shimazoe T, Nagashima M, Taniguchi I (2005) Electrocatalytic oxidation of glucose at gold nanoparticle-modified carbon electrodes in alkaline and neutral solutions. Electrochem Commun 7:189–193
Kohzuma T, Kikuchi M, Horikoshi N, Nagatomo S, Teizo Kitagawa A, Czernuszewicz RS (2006) Intersite structural rearrangement of the blue copper site induced by substrate binding: spectroscopic studies of a copper-containing nitrite reductase from Alcaligenes xylosoxidans NCIMB 11015. Inorg Chem 45:8474–8476
Huang Z, Zhu Z, Subhani Q, Yan W, Guo W, Zhu Y (2012) Simultaneous determination of iodide and iodate in povidone iodine solution by ion chromatography with homemade and exchange capacity controllable columns and column-switching technique. J Chromatog A. 1251:154–159
Kodamatani H, Yamazaki S, Saito K, Tomiyasu T, Komatsu Y (2009) Selective determination method for measurement of nitrite and nitrate in water samples using high-performance liquid chromatography with post-column photochemical reaction and chemiluminescence detection. J Chromatog A 1216:3163–3167
Wang S, Yin Y, Lin X (2004) Cooperative effect of Pt nanoparticles and Fe (III) in the electrocatalytic oxidation of nitrite. Electrochem Commun 6:259–262
Mori V, Bertotti M (1998) Amperometric detection with microelectrodes in flow injection analysis: theoretical aspects and application in the determination of nitrite in saliva. Talanta 47:651–658
Tian L, Liu L, Chen L, Lu N, Xu H (2005) Fabrication of amorphous mixed-valent molybdenum oxide film electrodeposited on a glassy carbon electrode and its application as a electrochemistry sensor of iodate. Sensor Actuat B: Chem. 105:484–489
Sun D, Zhu L, Huang H, Zhu G (2006) Fabrication of 9, 10-phenanthrenequinone/carbon nanotubes composite modified electrode and its electrocatalytic property to the reduction of iodate. J Electroanal Chem 597:39–42
Cheng H, Yin X, Xu Z, Wang X, Shen H (2011) A simple and demountable capillary microflow nebulizer with a tapered tip for inductively coupled plasma mass spectrometry. Talanta 85:794–799
Qian T, Yu C, Zhou X, Wu S, Shen J (2014) Au nanoparticles decorated polypyrrole/reduced graphene oxide hybrid sheets for ultrasensitive dopamine detection. Sensor Actuat B: Chem 193:759–763
Li Y, Niu X, Tang J, Lan M, Zhao H (2014) A comparative study of nonenzymatic electrochemical glucose sensors based on Pt-Pd Nanotube and Nanowire arrays. Electrochim Acta 130:1–8
Huang M, Wu Y, Hu W (2014) A facile synthesis of reduced graphene oxide-wrapped WO3 nanowire composite and its enhanced electrochemical catalysis properties. Ceram Int 40:7219–7225
Du J, Yue R, Yao Z, Jiang F, Du Y, Yang P, Wang C (2013) Nonenzymatic uric acid electrochemical sensor based on graphene-modified carbon fiber electrode. Colloids Surf A Physicochem Eng Asp 419:94–99
Huang X, Li Y, Chen Y, Wang L (2008) Electrochemical determination of nitrite and iodate by use of gold nanoparticles/poly (3-methylthiophene) composites coated glassy carbon electrode. Sensors Actuators B Chem 134:780–786
J.-E. Lee, Y.J. Jang, W. Xu, Z. Feng, H.-Y. Park, J.Y. Kim, D.H. Kim. PtFe nanoparticles supported on electroactive Au-PANI core@ shell nanoparticles for high performance bifunctional electrocatalysis. J Mater Chem A. 2017
Rao H, Min C, Ge H, Lu Z, Xin L, Ping Z, Wang X, Hua H, Zeng X, Wang Y (2017) A novel electrochemical sensor based on Au@PANI composites film modified glassy carbon electrode binding molecular imprinting technique for the determination of melamine. Biosens Bioelectron 87:1029–1035
Yang Z, Fang Z, Sheng J, Ling Z, Liu Z, Zhu J, Gao P, Ye J (2017) Optoelectronic evaluation and loss analysis of PEDOT:PSS/Si hybrid heterojunction solar cells. Nanoscale Res Lett 12:26
Mercante LA, Facure MHM, Sanfelice RC, Migliorini FL, Mattoso LHC, Correa DS. One-pot preparation of PEDOT: PSS-reduced graphene decorated with Au nanoparticles for enzymatic electrochemical sensing of H 2 O 2. Appl Surf Sci. 2017;407:162–70.
Abdiryim T, Ali A, Jamal R, Osman Y, Zhang Y (2014) A facile solid-state heating method for preparation of poly(3,4-ethelenedioxythiophene)/ZnO nanocomposite and photocatalytic activity. Nanoscale Res Lett 9:89
Ali A, Jamal R, Abdiryim T, Huang X (2017) Synthesis of monodispersed PEDOT/Au hollow nanospheres and its application for electrochemical determination of dopamine and uric acid. J Electroanal Chem 787:110–117
Selvaganesh SV, Mathiyarasu J, Phani K, Yegnaraman V (2007) Chemical synthesis of PEDOT–Au nanocomposite. Nanoscale Res Lett 2:546
Liu Z, Xu J, Yue R, Yang T, Gao L (2016) Facile one-pot synthesis of Au–PEDOT/rGO nanocomposite for highly sensitive detection of caffeic acid in red wine sample. Electrochim Acta 196:1–12
Liu Z, Lu B, Gao Y, Yang T, Yue R, Xu J, Gao L (2016) Facile one-pot preparation of Pd–Au/PEDOT/graphene nanocomposites and their high electrochemical sensing performance for caffeic acid detection. RSC Adv 6:89157–89166
Dehsari HS, Shalamzari EK, Gavgani JN, Taromi FA, Ghanbary S (2014) Efficient preparation of ultralarge graphene oxide using a PEDOT:PSS/GO composite layer as hole transport layer in polymer-based optoelectronic devices. RSC Adv 4:55067–55076
Diker H, Durmaz GB, Bozkurt H, Yeşil F, Varlikli C (2017) Controlling the distribution of oxygen functionalities on GO and utilization of PEDOT:PSS-GO composite as hole injection layer of a solution processed blue OLED. Curr Appl Phys 17:565–572
Dinesh B, Saraswathi R (2016) Enhanced performance of Pt and Pt–Ru supported PEDOT–RGO nanocomposite towards methanol oxidation. Int J Hydrogen Energ 41:13448–13458
Guo M, Chen J, Li J, Tao B, Yao S (2005) Fabrication of polyaniline/carbon nanotube composite modified electrode and its electrocatalytic property to the reduction of nitrite. Anal Chim Acta 532:71–77
Xue K, Zhou S, Shi H, Feng X, Xin H, Song W (2014) A novel amperometric glucose biosensor based on ternary gold nanoparticles/polypyrrole/reduced graphene oxide nanocomposite. Sensors Actuators B Chem 203:412–416
Abdiryim T, Jamal R, Zhao C, Awut T, Nurulla I (2010) Structure and properties of solid-state synthesized poly (3′, 4′-ethylenedioxy-2, 2′: 5′, 2 ″-terthiophene). Synthetic Met. 160:325–332
Abdiryim T, Ubul A, Jamal R, Xu F, Rahman A (2012) Electrochemical properties of the poly(3,4-ethylenedioxythiophene)/single-walled carbon nanotubes composite synthesized by solid-state heating method. Synthetic Met. 162:1604–1608
Geetha S, Trivedi D (2005) A new route to synthesize high degree polythiophene in a room temperature melt medium. Synthetic Met. 155:232–239
Zhang D, Qin J, Xue G (1999) An investigation of the electropolymerization of terthiophene in boron fluoride–ethyl ether. Synthetic Met 100:285–289
Yamamoto T, Shimizu T, Kurokawa E (2000) Doping behavior of water-soluble π-conjugated polythiophenes depending on pH and interaction of the polymer with DNA. React Funct Polym 43:79–84
Madl CM, Kariuki PN, Gendron J, Piper LF, Jones WE Jr (2011) Vapor phase polymerization of poly (3, 4-ethylenedioxythiophene) on flexible substrates for enhanced transparent electrodes. Synth Met 161:1159–1165
Madl CM, Kariuki PN, Gendron J, Piper LFJ, Jones WE (2011) Vapor phase polymerization of poly (3,4-ethylenedioxythiophene) on flexible substrates for enhanced transparent electrodes. Synth Met 161:1159–1165
Lu X, Zhang W, Wang C, Wen T-C, Wei Y (2011) One-dimensional conducting polymer nanocomposites: synthesis, properties and applications. Prog Polym Sci 36:671–712
Dinh NN, Chi LH, Thuy C, Thi T, Trung TQ, Truong V-V (2009) Enhancement of current-voltage characteristics of multilayer organic light emitting diodes by using nanostructured composite films. J Appl Phys 105:093518–093515
Jo S-H, Lee Y-K, Yang J-W, Jung W-G, Kim J-Y (2012) Carbon nanotube-based flexible transparent electrode films hybridized with self-assembling PEDOT. Synth Met 162:1279–1284
Shao Y, Zhang S, Wang C, Nie Z, Liu J, Wang Y, Lin Y (2010) Highly durable graphene nanoplatelets supported Pt nanocatalysts for oxygen reduction. J Power Sources 195:4600–4605
Augusto T, Teixeira Neto É, Teixeira Neto ÂA, Vichessi R, Vidotti M, de Torresi SIC (2013) Electrophoretic deposition of Au@PEDOT nanoparticles towards the construction of high-performance electrochromic electrodes. Solar Energ Mat Sol Cells 118:72–80
Chatraei F, Zare HR (2013) Nano-scale islands of ruthenium oxide as an electrochemical sensor for iodate and periodate determination. Mater Sci Eng: C 33:721–726
Xian H, Wang P, Zhou Y, Lu Q, Wu S, Li Y, Wang L (2010) Electrochemical determination of nitrite via covalent immobilization of a single-walled carbon nanotubes and single stranded deoxyribonucleic acid nanocomposite on a glassy carbon electrode. Microchim Acta 171:63–69
Acknowledgements
The authors are grateful to the National Natural Science Foundation of China (No. 21464014, No. 21564014).
Author information
Authors and Affiliations
Contributions
AA carried out the sample preparation and the experimental measure and participated in the study of material structures and the data analysis. RJ conceived the study, carried out the data analysis, and drafted the manuscript. TA coordinated the research and revised the manuscript. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ali, A., Zhang, Y., Jamal, R. et al. Solid-State Heating Synthesis of Poly (3,4-Ethylenedioxythiophene)/Gold/Graphene Composite and Its Application for Amperometric Determination of Nitrite and Iodate. Nanoscale Res Lett 12, 568 (2017). https://doi.org/10.1186/s11671-017-2338-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11671-017-2338-8