Abstract
An economic and effective Pt-based alloy cocatalyst has attracted considerable attention due to their excellent catalytic activity and reducing Pt usage. In this study, PtNi alloy cocatalyst was successfully decorated on the g-C3N4/GO hybrid photocatalyst via a facile chemical reduction method. The Eosin Y-sensitized g-C3N4/PtNi/GO-0.5% composite photocatalyst yields about 1.54 and 1178 times higher hydrogen evolution rate than the Eosin Y-sensitized g-C3N4/Pt/GO-0.5% and g-C3N4/Ni/GO-0.5% samples, respectively. Mechanism of enhanced performance for the g-C3N4/PtNi/GO composite was also investigated by different characterization, such as photoluminescence, transient photocurrent response, and TEM. These results indicated that enhanced charge separation efficiency and more reactive sites are responsible for the improved hydrogen evolution performance due to the positive synergetic effect between Pt and Ni. This study suggests that PtNi alloy can be used as an economic and effective cocatalyst for hydrogen evolution reaction.

A significant enhancement of photocatalytic H2 evolution is realized over the Eosin Y-sensitized g-C3N4/PtNi/GO composite with PtNi alloy as an efficient cocatalyst.
Similar content being viewed by others
Background
Sustainable and large-scale hydrogen evolution from water using solar energy is considered to be one of the promising method toward solving the energy crisis and environmental pollution [1, 2]. To achieve this goal, a visible-light response photocatalyst and an efficient cocatalyst are required [3,4,5]. Usually, loading noble metal Pt as an efficient cocatalyst is highly necessary for achieving a high hydrogen evolution rate [6,7,8]. However, Pt is rare and expensive, which hinders its practical application. Reducing the amount of Pt usage while simultaneously maintaining its excellent catalytic activity for hydrogen evolution is desired. Replacing part of Pt with transition metal (Ni, Co, Cu, Fe, etc.) to form a bimetallic alloy cocatalyst is a promising potential way for achieving excellent catalytic activity and reducing the use of Pt [9,10,11]. In some case, the catalytic performance of Pt-based bimetallic alloy cocatalyst is comparable with pure Pt due to the positive synergetic effect between the two metals. Therefore, a visible-light response photocatalyst loaded with a bimetallic alloy cocatalyst has been paid more attention in recently.
Yu et al. reported that PtCo or PtNi alloy cocatalyst-modified Cu2ZnSnS4 showed higher H2 production efficiency than the pure Pt loading Cu2ZnSnS4 [12]. Pt3Co bimetallic cocatalyst decorated CdS were prepared by Hu et al. and exhibited enhanced hydrogen evolution performance [13]. PtCo and/or PtFe loading Zn1 − xCd x S were also evaluated in previous studies [14, 15]. However, low visible-light photocatalytic performance of Cu2ZnSnS4 or high toxicity of Cd hinders their practical application on a large scale. Carbon nitride (g-C3N4) has attracted attention due to its low cost [16]. Han et al. reported that a H2 evolution rate of 960 μmol g−1 h−1 was obtained over the PtCo/g-C3N4 photocatalyst under λ > 400 nm irradiation [17]. PtNix/g-C3N4 hybird photocatalyst was also studied by Bi et al., and a H2 evolution rate of 8456 μmol g−1 h−1 was achieved under full spectral irradiation [18]. However, the visible-light photocatalytic performance for the bimetallic alloy cocatalyst-modified g-C3N4 photocatalyst is still a little bit low due to the wide band gap of 2.7 eV and bad electron transfer ability. Eosin Y-sensitized g-C3N4 can harvest a wide range of visible light [19, 20]. Graphene oxide (GO) possesses highly electron transporting property and has been widely used as an electron acceptor [21,22,23,24,25]. Combining g-C3N4 and GO can promote the electron transfer capability in g-C3N4 and thus improve the electron-hole pair’s separation to improve the photocatalytic performance for hydrogen evolution [26,27,28,29,30,31]. Lately, we reported an efficient Eosin Y-sensitized g-C3N4/Pt/GO composite photocatalyst for hydrogen evolution [23]. The expensive Pt cocatalyst plays one of the important role for the relatively high hydrogen production performance. In order to decrease the expensive Pt usage and further improve its visible photocatalytic performance, exploiting low-cost Eosin Y-sensitized g-C3N4/GO composite photocatalyst loaded with Pt-based alloy cocatalyst is useful.
Here, the Eosin Y-sensitized g-C3N4/PtNi/GO composite photocatalyst was prepared for hydrogen evolution from water. The highest hydrogen evolution rate of 5.89 mmol g−1 h−1 is obtained over the Eosin Y-sensitized g-C3N4/PtNi/GO photocatalyst, which is much higher than the Eosin Y-sensitized g-C3N4/Pt/GO and g-C3N4/Ni/GO composite samples. To the best our knowledge, there is no previous report that the Eosin Y-sensitized g-C3N4/PtNi/GO composite is employed for hydrogen production from water. The optimal molar ratio of Pt/Ni and amount of PtNi cocatalyst were screened out in detail. In addition, mechanism of enhanced photocatalystic performance for the g-C3N4/PtNi/GO composite was also investigated through different characterization methods.
Experimental Section
Synthesis of the g-C3N4
g-C3N4 powders were synthesized as described in previous study [32]. In a typical procedure, urea (8 g) was placed in an alumina crucible with a cover. The crucible was heated to 600 °C at a heating rate of 5 °C/min and held for 2 h in a tube furnace. After thermal treatment, the light yellow g-C3N4 powders were collected for further using.
Preparation of GO
GO was prepared using the modified Hummers’ method [33]. Nature graphite (10 g) and NaNO3 (5 g) were putted into a beaker. Then, 230 mL concentrated sulfuric acid were added, and the process must be as slow as possible. The above reaction was proceeded with stirring under ice-water bath. Next, 10 g KMnO4 was added into the mixture solution and reacted for 3 h. The temperature of solution was raised to 35 °C and was maintained for 4 h. Then, 460-mL distilled water was poured into the above solution and heated up to about 98 °C for 3 h. After reaction, a certain amount of H2O2 (30%) and concentrated hydrochloric acid were added under stirring with the purpose of removing excess KMnO4 and SO42−. Finally, the GO sample was obtained by freeze-drying for 24 h.
Synthesis of g-C3N4/Ni/GO, g-C3N4/Pt/GO, and g-C3N4/PtxNiy/GO Composite Photocatalysts
Synthesis of g-C3N4/PtNi/GO-X (X represents the weight ratio of PtNi cocatalyst to g-C3N4/GO composite and the molar ratio of Pt to Ni is 1:1): In a typical, 133 mg of g-C3N4 were dispersed into 50 mL anhydrous ethanol. Excess NaBH4 reductant was added into the mixture solution under stirring. Then, a certain volume of NiCl2·6H2O solution (0.1 mol/L) and H2PtCl6 solution (1.0 mmol/L) were dropwise added into the above solution. In order to investigate the added procedure of NiCl2·6H2O and H2PtCl6 solutions, three methods including simultaneous loading of Pt and Ni, loading of Pt and then Ni or in reverse were chose. Then, the suspension solution was stirred for 5 h to achieve a uniform dispersion for PtNi cocatalyst in the g-C3N4. After that, the g-C3N4/PtNi-X samples were collected by centrifugation to wash away excess NaBH4 for several times. Afterwards, 67 mg GO and the g-C3N4/PtNi-X sample were dispersed into 100-mL distilled water simultaneously. The suspension solution was ultrasonicated at 500 W for 10 h. After that, a series of g-C3N4/PtNi/GO-X composite photocatalysts were obtained by centrifuged and then dried at 60 °C in a vacuum oven for one night. Other bimetallic PtNi alloy cocatalysts with different Pt/Ni molar ratio (9:1, 3:1, 1:3, 1:9) were also prepared as same as the aforementioned method, which named as Pt9Ni1, Pt3Ni1, Pt1Ni3, and Pt1Ni9, respectively, specially, the 1:1 M ratio of Pt/Ni name as PtNi.
Synthesis of g-C3N4/Ni/GO-0.5% and g-C3N4/Pt/GO-0.5% samples (0.5% represents the weight ratio of Ni or Pt to the g-C3N4/GO composite): The g-C3N4/Ni/GO-0.5% and g-C3N4/Pt/GO-0.5% samples were prepared with the same preparation procedure of g-C3N4/PtNi/GO-X samples except for adding different volume of NiCl2·6H2O solution or H2PtCl6 solution. The 2:1 weight ratio of g-C3N4 to GO is chosen in all g-C3N4/Ni/GO, g-C3N4/Pt/GO, and g-C3N4/PtxNiy/GO composite samples according to our previous study [32].
Characterization Methods
The XRD patterns were obtained using an X-ray diffraction diffractometer (Bruker D8-Advance, Germany) with Cu-Kα radiation. TEM images of the samples were recorded through the transmission electron microscopy (JEM-2100, Japan). Surface chemical states of the photocatalysts were measured by X-ray photo-electron spectroscopy (XPS, AXISULTRA) with monochromatic Al Ka X-rays (1486.6 eV). The photoluminescence (PL) spectra were measured on a JY HORIBA FluoroLog-3 spectrometer, and the excited wavelength of 460 nm was chosen. The curves of photocurrent response were carried out in an electrochemical workstation (CHI660E, Chenhua, China) using a conventional standard three-electrode cell under visible light irradiation (λ > 420 nm). 0.1 mol/L Na2SO4 solution was used as electrolyte.
Photocatalytic Activity Measurement
Photocatalytic experiments were conducted in a Pyrex cell with a top flat window at 6 °C. Typically, 50 mg photocatalyst powder and 50 mg Eosin Y dye were added into 100 mL H2O containing 20 vol% (v/v) triethanolamine (TEOA, pH = 7). A 300-W xenon lamp (D59, Beijing China Education Au-light Co., Ltd) coupled with a UV cut-off filter (> 420 nm) was used as the light source. The amounts of hydrogen were measured through a gas chromatography (GC-7920, TCD, Ar carrier).
Results and Discussion
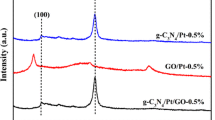
The XRD patterns of g-C3N4/Ni/GO-0.5%, g-C3N4/Pt/GO-0.5%, and g-C3N4/PtNi/GO-0.5% samples are shown in Fig. 1. Two obvious diffraction peaks are observed for the three samples. A small peak centered at 2θ = 13.8° is assigned to the (100) peak of g-C3N4, which arises from the in-plane structural packing motif [34]. The strong diffraction peak at 27.4° is indexed as the (002) peak of g-C3N4, which corresponds to the interlayer stacking of conjugated aromatic system [35]. For the three evaluated samples, no GO, Pt and/or Ni cocatalyst diffraction signal were detected. Sufficient exfoliation of GO in the composite can result in no GO information [28]. While the amount of Pt and/or Ni are too small to detect by XRD method. As shown in Fig. 2, the three different samples exhibit similar thinner laminar structure after ultrasonic treatment. An intimate contact formed between 2D-layered structure of g-C3N4 and the nanosheet structure of GO. In Fig. 2a, b, a bit larger of Ni or Pt nanoparticles are dispersed on the interlayer or surface of g-C3N4. Compared with pure Ni and Pt, the size of the PtNi alloy cocatalyst particles is reduced and the dispersion of PtNi alloy cocatalyst is improved (see Fig. 2c). The small size of PtNi alloy cocatalyst will provide more reactive sites for hydrogen evolution, and the high dispersion of PtNi alloy cocatalyst is benefited to the electrons transfer from g-C3N4 and/or GO to PtNi cocatalyst. The improved dispersion of PtNi alloy cocatalyst was also observed in previous study [36]. The precision reason need further investigate.
In order to investigate the surface chemical element and valence states of g-C3N4/Ni/GO, g-C3N4/Pt/GO, and g-C3N4/PtNi/GO samples, high-resolution XPS spectra of the three different samples were measured and the results are shown in Fig. 3. In Fig. 3a, the XPS spectrum of C 1 s can be fitted into two strong peaks with the binding energies at about 284.8 eV and 288.2 eV, which are assigned to C-C and N=C-N, respectively [37]. The two peaks are the characteristic of carbon species in g-C3N4. Two small peaks at 286.7 eV and 287.7 eV are also obtained, which belong to the C-O and C=O functional groups on the surface of GO, respectively [38]. In Fig. 3 (b), the characteristic peaks of C-N-C, N-(C)3, and C-N-H groups in g-C3N4 were detected, which are located at the binding energies of 398.7, 400.3, and 401.4 eV, respectively [39]. In Fig. 3 (c), the banding energies of O 1 s are found at 532.4 and 533.8 eV, which are assigned to oxygen-containing functional groups in the composite sample and surface adsorption of oxygen species, respectively [40]. Figure 3 (d) exhibits the XPS spectra of the Pt 4f doublet (4f7/2 and 4f5/2). The Pt 4f7/2 and 4f5/2 peaks are located at 70.97 and 74.28 eV for the g-C3N4/Pt/GO-0.5% sample, respectively, which represent the signal of Pt0 [41, 42]. For the g-C3N4/PtNi/GO-0.5% sample, the orbital binding energies of Pt 4f shift about 0.42 eV to high binding energy compared with pure Pt. The obvious peak shift suggests that Pt electron is slight loss, which indicates that the PtNi alloy cocatalyst is formed in the g-C3N4/PtNi/GO-0.5% sample. As shown in Fig. 3e, the binding energies at 852.54 and 870.18 eV can be assigned to Ni 2p3/2 and Ni 2p1/2 for the g-C3N4/Ni/GO-2% sample, respectively, which are the characterization signal of Ni0 [43]. Compared with g-C3N4/Ni/GO-2%, the binding energies of Ni 2p shifted to low binding energy for the g-C3N4/PtNi/GO-2% sample. The result suggests that a change in the surrounding environment of Ni atoms occurs, which further confirms that the PtNi alloy cocatalyst is successfully synthesized [41]. The exact molar ratio of Pt to Ni in the g-C3N4/PtNi/GO-0.5% sample is 9:11 through XPS measurement. Based on above analysis, it can be concluded that the g-C3N4/PtNi/GO composite with PtNi alloy as cocatalyst was obtained by combing a facile liquid-phase sonochemical way with a chemical reduction method.
Figure 4 shows the H2 evolution rate of series of g-C3N4/PtNi/GO-0.5% samples loaded with different type of cocatalyst. Simultaneous loading of Pt and Ni names as g-C3N4/PtNi/GO-0.5%; Loading of Pt and then Ni names as g-C3N4/Pt-Ni/GO-0.5%; Loading of Ni and then Pt names as g-C3N4/Ni-Pt/GO-0.5%. For Eosin Y-sensitized g-C3N4/Ni/GO-0.5% sample with pure Ni as cocatalyst, the H2 evolution rate is very low and only reach to 0.005 mmol g−1 h−1. After replacing Ni with Pt as cocatalyst, a significant increase of H2 evolution rate is observed, which sharply increases to 3.82 mmol g−1 h−1 for the Eosin Y-sensitized g-C3N4/Pt/GO-0.5% sample. The result suggests that loading efficient Pt cocatalyst is necessary to achieve excellent performance for H2 generation. When employed PtNi alloy as cocatalyst, the Eosin Y-sensitized g-C3N4/PtNi/GO-0.5% composite shows the highest H2 evolution rate of 5.89 mmol g−1 h−1, which is about 1.54 and 1178 times higher than the Eosin Y-sensitized g-C3N4/Pt/GO-0.5% and g-C3N4/Ni/GO-0.5% samples, respectively. The enhanced performance can be attributed to the positive synergistic effect between Pt and Ni. Compared with pure Pt cocatalyst, PtNi alloy cocatalyst accelerates the accumulation of photongenerated electrons, which will provide more electrons for hydrogen evolution [18]. In addition, the small size and high dispersion of PtNi alloy cocatalyst can provide more H2 evolution sites and enhance the electrons transfer, respectively. When loaded Pt and then Ni or in reverse, an obvious reduction of H2 evolution activity is observed. In fact, loading Pt and then Ni or in reverse does not form PtNi alloy cocatalyst [44]. The result indicates that achieving high H2 generation rate is strongly relied on employing efficient PtNi alloy cocatalyst.
The composition of PtNi alloy cocatalyst has an important effect on the catalytic activity for H2 evolution. Therefore, the H2 production rate of Eosin Y-sensitized g-C3N4/PtxNiy/GO-0.5% samples loaded with different Pt/Ni molar ratio were investigated and the results are shown in Fig. 5a. The H2 production rate is increased gradually with increasing Ni/Pt molar ratio. When the molar ratio of Ni/Pt is 1:1, the highest hydrogen production rate of 5.89 mmol g−1 h−1 is obtained. Further increasing the amount of Ni leads to a drop in the H2 evolution activity. The degradation of H2 generation performance may come from the reducing number of Pt active sites for H2 evolution. Pt active sites have stronger adsorption of hydrogen ions than Ni [12]. Therefore, H2 evolution preferentially takes places on Pt instead of Ni. Figure 5b shows the H2 production rate of Eosin Y-sensitized g-C3N4/PtNi/GO samples loaded with different amounts of PtNi alloy cocatalyst. When the weight content of PtNi alloy cocatalyst is 0.5%, a hydrogen evolution rate of 2.45 mmol g−1 h−1 is obtained for the g-C3N4/PtNi/GO-0.25% sample. The hydrogen evolution rate increases from 2.45 mmol g−1 h−1 to the highest value of 5.89 mmol g−1 h−1 after the amount of PtNi alloy cocatalyst up to 0.5%. In further increasing the amount of PtNi alloy cocatalyst, the hydrogen evolution performance shows a slight decrease. Excess PtNi alloy cocatalyst can hinder the light absorption of Eosin Y and g-C3N4 and thus degrade the photocatalytic performance. The stability of hydrogen generation for the g-C3N4/PtNi/GO-0.5% sample was also measured and the result is shown in Fig. 6. After 4 cycle test, the hydrogen generation rate for the g-C3N4/PtNi/GO-0.5% sample shows a slight decrease, which indicates that the g-C3N4/PtNi/GO-0.5% composite sample has relatively stability for hydrogen evolution. A strong covalent bond between the carbon and nitride in C3N4 and the weak degradation of Eosin Y are the two main reasons for the hydrogen evolution stability of g-C3N4/PtNi/GO-0.5% sample [19, 45].
a H2 production rate of g-C3N4/PtxNiy/GO-0.5% samples loaded with different Pt/Ni molar ratio. b H2 production rate of g-C3N4/PtNi/GO samples loaded with different amounts of PtNi alloy cocatalyst. Light source: 300 W Xenon lamp (λ > 420 nm). Reaction solution: 100 mL 20% (v/v) TEOA aqueous solution (pH = 7)
In order investigate mechanism for the improved photocatalystic performance of g-C3N4/PtNi/GO sample with PtNi alloy as cocatalyst, two possible reasons of light absorption and charge separation efficiency were evaluated. Figure 7 shows the UV-Vis diffuse reflectance spectra of g-C3N4/Ni/GO-0.5%, g-C3N4/Pt/GO-0.5%, and g-C3N4/PtNi/GO-0.5% samples. The three different samples exhibit an obvious absorption after about 450 nm, which is from the metal cocatalyst [18]. The g-C3N4/Ni/GO-0.5% sample with pure Ni as cocatalyst shows the strongest absorption after about 450 nm. However, the H2 evolution rate for the g-C3N4/Ni/GO-0.5% sample is the lowest. The result suggests that the improved H2 evolution performance is not coming from the enhanced light absorption.
Charge separation efficiency can be characterized by the photoluminescence (PL) quenching spectra [46, 47]. In general, a strong intensity of PL spectra indicates serious charge carrier recombination. Figure 8a shows the photoluminescence (PL) quenching spectra of Eosin Y by the g-C3N4/Ni/GO-0.5%, g-C3N4/Pt/GO-0.5%, and g-C3N4/PtNi/GO-0.5% samples. Only the Eosin Y solution without photocatalyst exhibits an extensive emission peak at about 540 nm because of Eosin Y’s conjugate xanthene structure and strong recombination capacity of photogenerated electron-hole pairs in excited Eosin Y. Obvious fluorescence quenching is observed after adding different type of photocatalysts into the Eosin Y solution. The fluorescence quenching suggests that the electrons transfer to photocatalysts from excited Eosin Y and then migrate to cocatalyst for proton reduction. In addition, there is a small blue shift (ca. 1.3 nm) of PL quenching spectra for the three different evaluated composite photocatalysts, which can be attributed to the noncovalent π-π stacking interaction among Eosin Y, g-C3N4 and GO [48]. The PL quenching spectra of g-C3N4/Pt/GO-0.5% sample shows a moderated intensity, which is lower than the g-C3N4/Ni/GO-0.5% sample. Importantly, the g-C3N4/PtNi/GO-0.5% sample exhibits the lowest fluorescence intensity, which implies that the PtNi alloy cocatalyst is the most efficient cocatalyst for improving the charge separation efficiency than pure Pt or Ni. The result is consistent with the H2 evolution activity (see Fig. 4). To further verify the charge transfer process, the transient photocurrent responses of g-C3N4/Ni/GO-0.5%, g-C3N4/Pt/GO-0.5%, and g-C3N4/PtNi/GO-0.5% samples were measured and the results are shown in Fig. 8b. The g-C3N4/PtNi/GO-0.5% sample exhibits the highest photocurrent response under visible-light irradiation (λ > 420 nm), which further confirms employing PtNi alloy cocatalyst is essential to improve charge separation efficiency. Based on above results, the improved H2 evolution activity for the Eosin Y-sensitized g-C3N4/PtNi/GO-0.5% composite is attributed to the enhanced charge separation efficiency.
According to above results and mechanism analysis, we propose a schematic diagram to understand the H2 evolution process for the Eosin Y-sensitized g-C3N4/PtNi/GO composite sample (Fig. 9). Under visible-light irradiation, the photogenerated electrons in the LUMO of excited Eosin Y transfer to g-C3N4 and/or GO and then to the PtNi alloy cocatalyst for protons reduction. Meanwhile, the photoexcited electrons in the CB of g-C3N4 also flow to the PtNi alloy cocatalyst for H2 evolution reaction. At the same time, the photogenerated holes or oxidized Eosin Y dyes directly oxide the TEOA sacrificial agent.
Conclusion
Ternary g-C3N4/PtNi/GO composite was synthesized by combing a facile liquid-phase sonochemical way with a chemical reduction method. The Eosin Y-sensitized g-C3N4/PtNi/GO-0.5% composite shows the highest hydrogen evolution rate of 5.89 mmol g−1 h−1, which is about 1.54 and 1178 times higher than the Eosin Y-sensitized g-C3N4/Pt/GO-0.5% and g-C3N4/Ni/GO-0.5% samples, respectively. The enhanced photocatalytic activity can be ascribed to the positive synergetic effect between Pt and Ni as well as more reactive sites, which leads to efficient photoexcited electron-hole pairs separation. This study demonstrates that PtNi alloy can be employed as an economic and efficient cocatalyst for photocatalytic hydrogen evolution.
References
Fujishima A, Honda K (1972) Electrochemical photolysis of water at a semiconductor electrode. Nature 238:37–38
Wang X, Maeda K, Thomas A, Takanabe K, Xin G, Carlsson J, Domen K, Antonietti M (2009) A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat Mater 8:76–80
Hou Y, Laursen A, Zhang J, Zhang G, Zhu Y, Wang X, Dahl S, Chorkendorff I (2013) Layered nanojunctions for hydrogen-evolution catalysis. Angew Chem Int Ed 52:3621–3625
Jin Z, Yang H (2013) Exploration of Zr-metal-organic framework as efficient photocatalyst for hydrogen production. Nanoscale Res Lett 12:539–549
Yuan Y, Chen D, Zhong J, Yang L, Wang J, Liu M, Tu W, Yu Z, Zou Z (2017) Interface engineering of a noble-metal-free 2D–2D MoS2/Cu-ZnIn2S4 photocatalyst for enhanced photocatalytic H2 production. J Mater Chem A 5:15771–15779
Li X, Yu J, Low J, Fang Y, Xiao J, Chen X (2015) Engineering heterogeneous semiconductors for solar water splitting. J Mater Chem A 3:2485–2534
Zhang X, Peng T, Song S (2016) Recent advances in dye-sensitized semiconductor systems for photocatalytic hydrogen production. J Mater Chem A 4:2365–2402
Li Y, Wang L, Liang J, Gao F, Yin K, Dai P (2017) Hierarchical heterostructure of ZnO@TiO2 hollow spheres for highly efficient photocatalytic hydrogen evolution. Nanoscale Res Lett 12:531–536
Takako H, Hiroyuki U, Masahiro W (1999) Enhancement of the electroreduction of oxygen on Pt alloys with Fe, Ni, and Co. J Electrochem Soc 146:3750–3756
Garciacontreras M, Fernandezvalverde S, Vargasgarcia J, Cortesjacome M, Toledoantonio J, Angeleschavez C (2008) Pt, PtCo and PtNi electrocatalysts prepared by mechanical alloying for the oxygen reduction reaction in 0.5M H2SO4. Int J Hydrog Energy 33:6672–6680
Qin L, Li X, Kang S (2013) Synergetic effect of Cu-Pt bimetallic co-catalyst on SrTiO3 for efficient photocatalytic hydrogen production from water. RSC Adv 00:1–3
Yu X, An X, Genç A, Ibáñez M, Arbiol J, Zhang Y, Cabot A (2015) Cu2ZnSnS4–PtM (M = Co, Ni) Nanoheterostructures for Photocatalytic Hydrogen Evolution. J Phys Chem C 119:21882–21888
Hu Z, Yu J (2013) Pt3Co-loaded CdS and TiO2 for photocatalytic hydrogen evolution from water. J Mater Chem A 1:12221
Shu D, Wang H, Wang Y, Li Y, Liu X, Chen X, Peng X, Wang X, Ruterana P, Wang H (2017) Composition dependent activity of Fe1−xPtx decorated ZnCdS nanocrystals for photocatalytic hydrogen evolution. Int J Hydrog Energy 42:20888–20894
Wang H, Li Y, Shu D, Chen X, Liu X, Wang X, Zhang J, Wang H (2016) CoPtx-loaded Zn0.5Cd0.5S nanocomposites for enhanced visible light photocatalytic H2 production. Int J Energy Res 40:1280–1286
Wen J, Xie J, Chen X, Li X (2017) A review on g-C3N4-based photocatalysts. Appl Surf Sci 391:72–123
Han C, Lu Y, Zhang J, Ge L, Li Y, Chen C, Xin Y, Wu L, Fang S (2015) Novel PtCo alloy nanoparticle decorated 2D g-C3N4 nanosheets with enhanced photocatalytic activity for H2 evolution under visible light irradiation. J Mater Chem A 3:23274–23282
Bi L, Gao X, Ma Z, Zhang L, Wang D, Xie T (2017) Enhanced separation efficiency of PtNix/g-C3N4 for photocatalytic hydrogen production. Chem Cat Chem 9:1–8.
Min S, Lu G (2012) Enhanced electron transfer from the excited Eosin Y to mpg-C3N4 for highly efficient hydrogen evolution under 550 nm irradiation. J Phys Chem C 116:19644–19652
Xu J, Li Y, Peng S, Lu G, Li S (2013) Eosin Y-sensitized graphitic carbon nitride fabricated by heating urea for visible light photocatalytic hydrogen evolution: the effect of the pyrolysis temperature of urea. Phys Chem Chem Phys 15:7657–7665
Yeh T, Chen S, Yeh C, Teng H (2013) Tuning the electronic structure of graphite oxide through ammonia treatment for photocatalytic generation of H2 and O2 from water splitting. J Phys Chem C 117:6516–6524
Xiang Q, Yu J (2013) Graphene-based photocatalysts for hydrogen generation. J Phys Chem Lett 4:753–759
Sun S, Wang W, Zhang L (2013) Bi2WO6 quantum dots decorated reduced graphene oxide: improved charge separation and enhanced photoconversion efficiency. J Phys Chem C 117:9113–9120
Li X, Yu J, Wageh S, Al-Ghamdi A, Xie J (2016) Graphene in photocatalysis: a review. Small 12:6640–6696
Yuan Y, Chen D, Zhong J, Yang L, Wang J, Yu Z, Zou Z (2017) Construction of a noble-metal-free photocatalytic H2 evolution system using MoS2/reduced graphene oxide catalyst and zinc porphyrin photosensitizer. J Phys Chem C 121:24452–24462
Wan W, Yu S, Dong F, Zhang Q, Zhou Y (2016) Efficient C3N4/graphene oxide macroscopic aerogel visible-light photocatalyst. J Mater Chem A 4:7823–7829
Tong Z, Yang D, Shi J, Nan Y, Sun Y, Jiang Z (2015) Three-dimensional porous aerogel constructed by g-C3N4 and graphene oxide nanosheets with excellent visible-light photocatalytic performance. ACS Appl Mater Interfaces 7:25693–25701
Liao G, Chen S, Quan X, Yu H, Zhao H (2012) Graphene oxide modified g-C3N4 hybrid with enhanced photocatalytic capability under visible light irradiation. J Mater Chem 22:2721–2726
Pu C, Wan J, Liu E, Yin Y, Li J, Ma Y, Fan J, Hu X (2017) Two-dimensional porous architecture of protonated GCN and reduced graphene oxide via electrostatic self-assembly strategy for high photocatalytic hydrogen evolution under visible light. Appl Surf Sci 399:139–150
Xiang Q, Yu J, Jaroniec M (2011) Preparation and enhanced visible-light photocatalytic H2-production activity of graphene/C3N4 Composites. J Phys Chem C 115:7355–7363
Aleksandrzak M, Kukulka W, Mijowska E (2017) Graphitic carbon nitride/graphene oxide/reduced graphene oxide nanocomposites for photoluminescence and photocatalysis. Appl Surf Sci 398:56–62
Wang P, Guan Z, Li Q, Yang J (2018) Efficient visible-light-driven photocatalytic hydrogen production from water by using Eosin Y-sensitized novel g-C3N4/Pt/GO composites. J Mater Sci 53:774–786
Offeman W (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339
Han Q, Wang B, Gao J, Cheng Z, Zhao Y, Zhang Z, Qu L (2016) Atomically thin mesoporous nanomesh of graphitic C3N4 for high-efficiency photocatalytic hydrogen evolution. ACS Nano 10:2745–2751
Niu P, Zhang L, Liu G, Cheng H (2012) Graphene-like carbon nitride nanosheets for improved photocatalytic activities. Adv Funct Mater 22:4763–4770
Yang M, Dang Q, Xie Z, Guo H (2011) Study on CH4 Conversion over Ce-Zr solid solution supported nano Ni-Pt bimetallic catalysts: preparation and characterization of catalysts. Mater Sci Forum 694:319–323
Tay Q, Kanhere P, Ng C, Chen S, Chakraborty S, Huan A, Sum T, Ahuja R, Chen Z (2015) Defect engineered g-C3N4 for efficient visible light photocatalytic hydrogen production. Chem Mater 27:4930–4933
Wang Z, Du Y, Zhang F, Zheng Z, Zhang X, Feng Q, Wang C (2013) Photocatalytic degradation of pendimethalin over Cu2O/SnO2/graphene and SnO2/graphene nanocomposite photocatalysts under visible light irradiation. Mater Chem Phys 140:373–381
Cao S, Yuan Y, Barber J, Loo S, Xue C (2014) Noble-metal-free g-C3N4/Ni(dmgH)2 composite for efficient photocatalytic hydrogen evolution under visible light irradiation. Appl Surf Sci 319:344–349
Ong W, Tan L, Chai S, Yong S (2015) Graphene oxide as a structure-directing agent for the two-dimensional interface engineering of sandwich-like graphene-g-C3N4 hybrid nanostructures with enhanced visible-light photoreduction of CO2 to methane. Chem Commun (Camb) 51:858–861
Cao N, Yang L, Dai H, Liu T, Su J, Wu X, Luo W, Cheng G (2014) Immobilization of ultrafine bimetallic Ni-Pt nanoparticles inside the pores of metal-organic frameworks as efficient catalysts for dehydrogenation of alkaline solution of hydrazine. Inorg Chem 53:10122–10128
Liu Z, Lin X, Lee J, Zhang W, Han M, Gan L (2002) Preparation and characterization of platinum-based electrocatalysts on multiwalled carbon nanotubes for proton exchange membrane fuel cells. Langmuir 18:4054–4060
Zhen W, Gao H, Li Z, Ma J, Lu G (2016) Fabrication of low adsorption energy Ni-Mo clusters co-catalyst in metal-organic frameworks for visible photocatalytic hydrogen evolution. ACS Appl Mater Interfaces 8:10808–10819.
Qin L, Si G, Li X, Kang S (2013) Synergetic effect of Cu-Pt bimetallic co-catalyst on SrTiO3 for efficient photocatalytic hydrogen production from water. Roy Soc Chem 00:1–3
Theodore L, Theresa M, Du P, Luo G, Brian L, Eisenberg R (2009) Making hydrogen from water using a homogeneous system without noble metals. J Am Chem Soc 131:9192–9194
Min S, Lu G (2012) Sites for high efficient photocatalytic hydrogen evolution on a limited-layered MoS2 cocatalyst confined on graphene sheets-the role of graphene. J Phys Chem C 116:25415–25424
Zhang N, Shi J, Niu F, Wang J, Guo L (2015) A cocatalyst-free Eosin Y-sensitized p-type of Co3O4 quantum dot for highly efficient and stable visible-light-driven water reduction and hydrogen production. Phys Chem Chem Phys 17:21397–21400
Zhang W, Li Y, Peng S, Cai X (2014) Enhancement of photocatalytic H2 evolution of eosin Y-sensitized reduced graphene oxide through a simple photoreaction. Beilstein J Nanotech 5:801–811
Acknowledgements
The authors gratefully acknowledge financial support from the National Natural Science Foundation of China (51702087, 21673066, and 21703054), Program for Science & Technology Innovation Talents (15HASTIT043) and Innovative Research Team (16IRTSTHN015) from the University of Henan Province.
Author information
Authors and Affiliations
Contributions
This manuscript was written by PW. The preparation and characterization of samples were conducted by PW. The analysis and discussion of the results are carried out by PW, ZG, QL, LZ, JY. All authors revised and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wang, P., Zong, L., Guan, Z. et al. PtNi Alloy Cocatalyst Modification of Eosin Y-Sensitized g-C3N4/GO Hybrid for Efficient Visible-Light Photocatalytic Hydrogen Evolution. Nanoscale Res Lett 13, 33 (2018). https://doi.org/10.1186/s11671-018-2448-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11671-018-2448-y