Abstract
Graphite carbon nitride (g-C3N4) is well known as one of the most promising materials for photocatalytic activities, such as CO2 reduction and water splitting, and environmental remediation through the removal of organic pollutants. On the other hand, carbon nitride also pose outstanding properties and extensive application forecasts in the aspect of field emission properties. In this mini review, the novel structure, synthesis and preparation techniques of full-bodied g-C3N4-based composite and films were revealed. This mini review discussed contemporary advancement in the structure, synthesis, and diverse methods used for preparing g-C3N4 nanostructured materials. The present study gives an account of full knowledge of the use of the exceptional structural and properties, and the preparation techniques of graphite carbon nitride (g-C3N4) and its applications.
Similar content being viewed by others
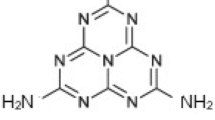
Introduction
The central energy source elated from the extraterrestrial space, solar energy capacities to surpass the almanac world’s energy request by a large border [1]. Given the long forecast era of the Sun, solar energy is also considered the ultimate renewable source that can be harvested on the planet, Earth [2, 3]. The unending and discontinuous nature of this energy source, however, presents key challenges in relationships of harvesting, storage, and utilization [4]. At the moment, there are a measure of technologies in place that may be used to face them. Solar energy can be flexibly gathered, transformed and kept in the form of heat, which can either distribute heat to residence or be further converted into electricity, as well as into other forms of energy [5]. The most innovative investigated technologies concerning solar photon gaining may be on those by the photocatalysis, as described by Edmond Becquerel, 1839 [5].
Predominantly, wastewater is the major source of pollution, specifically, wastewater produced due to chemical industrialization, because this wastewater contains pronounced concentration of large organic fragments which are tremendously poisonous and carcinogenic in nature [3]. Previously, the environmental remediation technology (which comprises of adsorption, biological oxidation, chemical oxidation, and incineration) has been used in the treatment of all types of organic and toxic wastewater and also has its effective application in solar energy utilization, environmental treatment, and biomedical and sensing applications. Fujishima and Honda revealed the exceptional knowledge about the photochemical splitting of water into hydrogen and oxygen in the presence of TiO2 in 1972; research interest has been focused in heterogeneous photocatalysis [3,4,5]. The speeding up of photoreaction in the existence of a catalyst is described as photocatalysis. Photocatalysis reaction is best known to be carried out in media such as gas phase, pure organic liquid phases, or aqueous solutions. Also, in most chemical degradation methods, photocatalytic degradation vis-à-vis photons and a catalyst is often identified as the best in controlling of organic wastewater, solar energy utilization, environmental treatment, and biomedical and sensing applications [3, 5]. Hence, the utmost technology used for the treatment of organic wastewater and related applications is attributed to the evolving solar light-driven photocatalysts [3].
Semiconductor photocatalysts can be used for the removal of ambient concentrations of organic and inorganic species from aqueous or gas phase systems in drinking water treatment, environmental tidying, and industrial and health applications. This is due to the massive ability of these semiconductors (g-C3N4, TiO2- and ZnO) to oxidize organic and inorganic substrates in air and water through redox processes for its effective application in solar energy utilization, wastewater, and environmental treatment, biomedical and sensing applications without any second pollution.
Polymeric graphitic carbon nitride (g-C3N4) has become the prime center for consideration in photocatalysis research [6]. g-C3N4 is a visible-light-response element with band gap of 2.7 eV, and the energy location of CB and VB is at − 1.1 and 1.6 eV via normal hydrogen electrode respectively [Wang et al. 2009]. In addition, g-C3N4 has the ability to resist attacks from heat, strong acid, and strong alkaline solution [7]. g-C3N4 has a unique ability to be simply prepared by thermally polycondensing the cheap N-rich precursors, such as dicyanamide, cyanamide, melamine, melamine cyanurate, and urea, and this is unlike the other metal-containing photocatalysts that require costly metal salts for preparation [6, 8]. Thermal condensation, solvothermal, chemical vapor deposition, microwave-assisted, polymerization, and hydrothermal synthesis are examples of preparative strategies (Table 2) which have been commendably applied in the preparation of carbon nitride for distinctive purposes and analysis in the area of photocatalysis and others [9].
Due to these outstanding properties of g-C3N4, the use of this promising g-C3N4 in water splitting, CO2 photo reduction, organic contaminants purification, catalytic organic synthesis, and fuel cells is more efficient and effective [6]. The number of admirable researches and reviews on g-C3N4 structure and preparation in the last few years has increased tremendously [10]. Authors mainly laid emphasis on the most contemporary advances on the structure, synthesis, and preparation techniques of g-C3N4 and carbon nitride (CNx) films vividly in this concise mini review. The unique structure and the novel synthesis and preparation techniques of g-C3N4, and CNX films are nicely presented, and the enlightened concepts on extending the preparation of g-C3N4 in this mini review are then emphasized. Also, the authors discussed the applications on g-C3N4, and the perspectives in future researches were also advocated.
Review
Graphitic Carbon Nitride and Photocatalysis
Photocatalysis is best referred to the acceleration of chemical conversions (oxidations and reductions) brought about through the activation of a catalyst. This reaction involves a semiconductor either alone or in combination with metal/organic/organometallic promoters, through light absorption, following charge or energy transfer to be adsorbed which can lead to the photocatalytic transformation of a pollutant. During a photocatalysis mechanism, there is a simultaneous occurrence of at least two main actions which aids a successful production of reactive oxidizing species (Fig. 2). These reactions are oxidation of dissociatively adsorbed H2O mostly generated by photogenerated holes and reduction of an electron acceptor also created by photoexcited electrons (Fig. 2). Hence, these reactions produce a hydroxyl and superoxide radical anion, respectively [11]. During photocatalysis reaction, it is obvious that there is photon-assisted generation of catalytically active species instead of the action of light as a catalyst in a reaction [12,13,14,15, 16]. Considerably, reaping of visible light, mostly from sunlight, by catalyst (photocatalyst) to initiate chemical transformations (Fig. 1) is described as photocatalysis. Application of C3N4 photocatalyst for wastewater treatment, solar energy utilization, environmental treatment, and biomedical and sensing applications has been discussed in many areas of science.
Enlightenment of a semiconductor catalyst, such as TiO2, ZnO, ZrO2, and CeO2, with photons carrying energy equal or in excess of its band gap, creating an electron hole pair similar to photo-induced electron transfer and absorption of light promotes one electron into the conduction band. The oxide may transfer its electron (Fig. 2) to any adsorbed electron acceptor (thereby promoting its reduction), while the hole (or the electron vacancy) may accept an electron from an adsorbed donor (promoting its oxidation). g-C3N4 is capable of catalyzing hydrogen/oxygen evolution and CO2 reduction under band gap excitation and in the presence of suitable co catalysts and/or sacrificial agents.
Schematic illustration of organic heterojunction formed between g-C3N4 and S-doped g-C3N4. Reproduced from Ref. [115]. Copyright 2015. Elsevier
Graphitic Carbon Nitride Nano-Based Particle
Materials with 1D nanostructures having distinct electronic, chemical, and optical properties could have their size and morphology adjusted. This ability of the 1D nanostructured materials has led to a novel advancement of diverse approaches to improve their photocatalytic activity [17]. In addition, there is guidance of electron movement in the axial direction and lateral confinement of electrons by these 1D nanostructures. There has been advancement of 2D materials from graphene to metal oxide and metal chalcogenide nanosheets and then to 2D covalent organic frameworks (g-C3N4).
The appropriate means of selection of precursors and condensation methods had led to two main types of g-C3N4 structural polymorphs and this includes, firstly, the g-C3N4 consist of a condensed s-triazine units (ring of C3N3) with a periodic array of single-carbon vacancies. The second type of g-C3N4 consists of the condensed tri-s-triazine (tri-ring of C6N7) subunits coupled through planar tertiary amino groups, and this has greater periodic vacancies in the lattice. The g-C3N4 networks mainly consists of melon-based segments (the second type structure; this consists of the tri-s-triazine unit, Fig. 3a) which is thermodynamically more stable compared to the melamine-based arrangements (the first type structure; this compose of the s-triazine, Fig. 3b) as described by the functional theory (DFT) calculations [18]. Hence, it is broadly believed that the tri-s-triazine nucleus is the fundamental building blocks for the formation of the g-C3N4 network.
Structure of Graphitic Carbon Nitride Nano-Based Particle
g-C3N4 are a class of two-dimensional (2D) polymeric materials comprising entirely of covalently linked, sp2-hybridized carbon and nitrogen atoms. Carbon and nitrogen have the distinction of various valence states forming bonding; therefore, in g-C3N4, there are diverse of valence bond structures. Research works have initiated that some C3N4 defect structures and amorphous structures of g-C3N4 are still the metastable structures, but with the upturn of N vacancy, these two kinds of configuration of g-C3N4 material usually lessen in bulk modulus. The structural characteristics, composition of materials, and crystallinity of g-C3N4 can be characterized and evaluated by XRD, XPS, and Raman techniques. In 1830, Berzelius described the general formula (C3N3H) n and Liebig also devised the notation “melon,” and these predictions had then led to more research focused on carbon nitride oligomers and polymers [19, 20]. Furthermore, these crystal structures have been found and stated in experiments [21,22,23]. The α-C3N4 is earlier found by Yu and coworkers [24]. A graphite-like loaded 2D structure of the graphite C3N4 is usually observed as nitrogen heteroatom- substituted graphite framework which mainly includes p-conjugated graphitic planes, and it is with sp2 hybridization of carbon and nitrogen atoms. Crystalline graphite is 3% less dense than the g-C3N4. Shifting the localization of electrons and then consolidating the bonds that is between the layers due to the nitrogen heteroatom substitution can help enlighten the interlayer distance of g-C3N4 [25].
Electronic Structure and Properties of g-C3N4
Currently, g-C3N4 is considered as a new-generation photocatalyst to recover the photocatalytic activity of traditional photocatalysts like TiO2, ZnO, and WO3. g-C3N4 is assumed to have a graphitic-like structure [26,27,28, 29, 30]. Thermal polycondensation method is generally used to prepare g-C3N4 and, hence, to investigate the electronic structure of g-C3N4.
The α-C3N4 is earlier found by Yu and coworkers [24]. These scientists used the calculation procedure of quantum mechanics clusters model and developed α-C3N4 by optimization the electronic structure of g-C3N4 for photocatalysis and others. In the structure of alpha-C3N4, C and N atoms linked by sp3 key was to used design the tetrahedron structure of g-C3N4. Liu and Cohen anticipated the existence of beta-C3N4 by means of band concept of first principles and prepared beta-C3N4 based on β-Si3N4 electronic structure. Liu and Cohen then revealed that the structure of β-C3N4 was hexagonal encompassing 14 atoms for each unit cell.
The outstanding prediction anticipated by Liu and Cohen in 1989 that the b-polymorph C3N4 would have exceptional high hardness values in comparison with diamond has enthused scientific research to date [26]. In 1993, C3N4 thin films via magnetron snorting of a graphite target on Si (100) and polycrystalline Zr substrates under a pure nitrogen ambience and consideration of the structure of C3N4 with analytical electron microscopy and Raman spectroscopy were synthesized by Chen and co-authors [27, 31]. Scientists, Teter and Hemley [28], foretold that alpha-C3N4, beta-C3N4, cubic-C3N4, pseudo cubic-C3N4, and graphite C3N4 show pronounced hardness approaching that of diamond in their experiment which they performed 3 years later as already described in accordance with first-principle calculations of the relative stability, structure, and physical properties of carbon nitride polymorphs.
Wang and coworkers [26, 32] applied ab initio evolutionary algorithm structure searches to calculate the precise structure of g-C3N4 prepared by thermal polycondensation and salt-melt synthesis methods for an enhanced visible-light-responsive photocatalysis. The most stable structure 1–3 were predicted for heptazine-based g-C3N4. The order of phase stability was 1 > 2 > 3. Contrary to other layered structures, distorted phases in heptazine-based g-C3N4 (see Fig. 3) were the most stable. This structure contributes the enhanced photocatalytic activity of the promise. In g-C3N4, lone pair electrons of nitrogen are mostly accountable for band structure and development of valence band.
Preparation of Graphitic Carbon Nitride Nano-Based Particle
Synthesis
The interesting tribological and electronic nature of graphitic carbon nitrides makes it possible to develop a method to deposit layers of graphitic carbon nitrides in a controlled manner; hence, graphene nitride can be obtained. Considerably, the benchmark particle for comparison is the bulky g-C3N4. This particle can be best achieved by using selection of nitrogen-rich precursors without direct C–C bonding, such as cyanamide, dicyandiamide, melamine, thiourea, urea, or mixtures through various preparative methods (Tables 1, 2, and 3), for instant, thermal condensation [33]. Carbon nitride materials are mostly bulk resources with small surface area, usually less than 10 m2 g−1 when they are prepared or synthesized by direct condensation of the nitrogen-containing organic precursors [34].
Mesoporous structure, when mineralized and the specific surface area amplified, helps to fine-tune the physicochemical properties and then increases the photocatalytic performance of graphite carbon nitride (g-C3N4). Nano-casting/replication of mesoporous silica matrices is the first method used to prepare graphite carbon nitride (g-C3N4), these were famous for their cohort of the corresponding carbon nanostructures [35]. Great efforts were then put in place to bring out more innovative schemes for g-C3N4 modification, which was enthused by the hard template method (Table 1). Liu and Cohen then discovered the (Table 1) soft template technique [26], and the other g-C3N4 modification schemes such as acidic solution impregnation, the ultrasonic dispersion method, and chemical functionalization [26] were also discovered. These methods as described above were good signs of the principle in modifying the surface chemical properties and the texture of g-C3N4, alone with its electronic potentials.
Thermal treatments, such as physical vapor deposition (PVD) [36], chemical vapor deposition (CVD) [37], solvothermal method [38], and solid-state reaction [38], are used for polymerizing plentiful nitrogen-rich and oxygen-free compound precursors comprising pre-bonded C–N core structures (triazine and heptazine derivatives), and these serve as the basic techniques for graphite carbon nitride (g-C3N4) synthesis. The commonly used precursors for the preparation of graphite carbon nitride (g-C3N4) through polymerization include cyanamide [39], dicyandiamide [40], melamine [41], urea [42], thiourea [43], guanidinium chloride [44], and guanidine thiocyanate [45]. The use of accomplished elements directly is actually challenging in many areas; this is due to the weak dispersity and ordinary nature of the bulk g-C3N4. The use of ample micro/nanostructures and morphologies to prepare different kinds of g-C3N4 has been intensely researched by scientist over the few last years of photocatalysis studies. For example, ultrathin g-C3N4 nanosheets which were prepared by exfoliating bulk g-C3N4 materials [46,47,48] were negatively charged and could be well dispersed in water.
Thermal oxidation exfoliation, ultrasonic exfoliation, and chemical exfoliation are well known as the major exfoliation methods used for preparing g-C3N4 materials. Meso-g-C3N4 materials have great performances such as great photocatalytic activity due to their greater specific surface area (up to 830 m2 g−1) and larger porosity (up to 1.25 cm3 g−1); also, the larger numbers of active sites present on the surface and higher size or shape selectivity enhances their excellent performances. The utmost essential pathways for the preparation of meso-g-C3N4 include soft templating (self-assembly) [49, 50] and hard templating (nano-casting) [51] methods (Table 1 and Fig. 4). Smaller sizes, popularly known as g-C3N4 quantum dots (QDs), were used by a lot of great research scientists in their researches for the synthesis of g-C3N4 [52,53,54,55]. Two main approaches to synthesize 2D g-C3N4 nanosheets are delamination of layered g-C3N4 solids into free-standing nanosheets mostly known as top–down strategy (Fig. 5) and the anisotropic assembly of organic molecules in a 2D manner (Fig. 6), also called bottom–up strategy. [56] Remarkably for the diverse chemical structure and electronic band structure of the CN nanosheets, the as-prepared CN nanosheets revealed a unique electrochemiluminescence (ECL) emission response to numerous metal-ions. Due to this, there has been a successful development of ECL sensor with rapid detection of numerous metal-ions.
TEM images of TCN (a and b) and MCN (c and d) using a hard templating approach. Reproduced with permission from [120]. Copyright 2015. Elsevier
Schematic illustration of synthesizing CNNs by using the top–down and bottom–up strategies (reproduced from ref. [121] with permission from The Royal Society of Chemistry)
Schematic diagram of Preparation and Enhanced Visible-light Photocatalytic by the decrease of RhB by different photocatalysts as a function of visible light irradiation time (photocatalysts loading, 0.5 g/L; initial RhB concentration, about 10 mg/L, without pH modulation). The photocatalysts used were pure g-C3N4 and a series of g-C3N4/ BiOCl hybrids, b cyclic degradation of RhB over BC3, c XRD patterns of BC3 photocatalysts before and after the photocatalytic process, and d plots of TOC versus degradation time. (Reproduced from ref. [122] with permission from Springer-Verlag GmbH Germany 2017)
Techniques Used in Preparing Graphitic Carbon Nitride Nano-Based Particle
The study on the syntheses of carbon nitride (g-C3N4 and CNx) has enthused the curiosity of researchers from all over the world. g-C3N4 and films with precise photocatalytic properties have been synthesized [57, 58]. Thermal condensation, solvothermal, chemical vapor deposition, microwave-assisted, polymerization, and hydrothermal synthesis approached are methods (Table 2) which have been effectively used in the preparation of carbon nitride for different purposes and analysis in the area of photocatalysis and others [9].
Thermal and Solvothermal Treatment Methods
Based on polycondensation reaction between melamine and cyanuric chloride in the presence of nickel powder, Li and research team [41] proposed two major methods for the synthesis of nitrogen-rich graphitic carbon nitrides. These two methods were solvothermal methods using benzene as solvent and solvent-free solid reaction way with thermal treatment (Fig. 7). Other works by many scientists [59,60,61,62, 63] suggested that solvothermal reactions usually produce crystalline after washing and drying, and do not require post annealing treatment. These scientists also proposed enhanced photocatalytic activities with this method (Fig. 8).
SEM images of sample B: (a) alumina particles coated with carbon nitride; (b) detail of the projecting indentations of carbon nitride. It is possible to observe the jaggy shape of the carbon nitride sheets obtained by pyrolysis. SEM images of sample A: (c) and (d) views of alumina particles coated with carbon nitride. Reproduced from [60]
TEM images and an electron diffraction pattern of mp-C3N4 after removal of the silica nanoparticles. Reproduced with permission [123]. Copyright John Wiley & Sons Inc., 2006
Niu and co. also reported the morphological changes when solvothermal technique was used [64]. Loumagne and coworkers [65] testified the physicochemical possessions of SiC-based deposits, achieved via the thermal decomposition of CH3SiCl3 in hydrogen. Kelly and group [66] reported synthesis of TaC by using reactants tantalum (V) chloride and carbon mixed under an argon-filled glove box through the thermal process. Successively, thermal condensation method, which mostly consists of conjugated aromatic heptazine system with graphitic assembling characteristics, has been used several moments to prepare g-C3N4 [36]. The use of solvothermal technique for g-C3N4 synthesis has great remunerations such as even and fine particle formation, little energy consumption, and higher economic feasibility as compared to the outdated thermal condensation method. Conversely, these methods are still time-consuming, demanding to a certain extent of a few hours to complete particle formation and crystallization.
Chemical Vapor Deposition
Investigation by Roberto and coworkers [60] suggested the use of chemical vapor deposition (CVD) for graphitic carbon nitride synthesis by the reaction between melamine and uric acid has high photocatalytic activity. It was found that the formed graphitic carbon nitride was with a structure based on heptazine blocks.
Roberto and coworkers then proposed that these carbon nitrides’ nature revealed a jaggy-like shape (Fig. 7), crystallinity, and a nanometric texture. Kelly et al. [66] has reported the synthesis of TaC by using reactants tantalum (V) chloride and carbon mixed under an argon-filled glove box via thermal technique and later transformed to TaC nanoparticles via chemical technique. CVD is one of the most useful methods to prepare monolayer graphene of high structural quality for use in different devices for catalytic activities [67]. Wang and group [26, 32] obtained CN푥 films on Ni substrate by using HFCVD method firstly. Because the preparation of these films is more likely to produce C–H and N–H linkage under the CVD conditions, most of the CN푥 films are amorphous. From previous studies, CVD procedures are used to prepare carbon nitrides, the choice of substrate materials is very critical to be considered. Large area samples can be prepared by exposing a metal to different hydrocarbon precursors at high temperatures. There are different types of CVD methods available such as plasma-enhanced CVD, thermal CVD, and hot/cold wall CVD. CVD methods mainly consist of electron cyclotron resonance, hot filament-assisted, DC glow discharge, radiofrequency discharge, and microwave plasma chemical vapor deposition. Bias of auxiliary hot filament chemical vapor deposition (HFCVD) is one of the local tools used in the deposition of diamond films and others. The exact mechanism of the formation of graphene depends on the growth substrate but typically initiates with the growth of carbon atoms that nucleate on the metal after decomposition of the hydrocarbons, and the nuclei grow then into large domains [68]. Recently, produced high-quality monolayer graphene by using resistive heating cold wall CVD was also 100 times faster than conventional CVD.
Sol–Gel Synthesis
Sol–gel synthetic technique is a process through which a solid product or a nano-material is formed from a solution after the transformation of the gel intermediate. In this synthesis method, reactants are mixed at the molecular level allowing fast reactions and lead to more homogeneous products with higher surface area. Remarkably, this technique has been used to synthesize different types of nanoparticles including metal carbide, and nitride processes for photocatalysis [69]. The synthesis of metal nitride using sol–gel processes can be traced back to the use of metal-organic compounds (synthesized from metal element and dialkylamine) [70].
Microwave Heating
In recent times, microwave heating has been used widely for the preparation of fine chemicals and pharmaceuticals as compared to the methods described above, because it permits comprehensive reaction range and short reaction time, which are appropriate for production on an industrial scale [71]. A simple-minded technique was adopted by Wang and coworkers to synthesize g-C3N4 using a cheap/less-expensive nitrogen-rich precursor which can then be active as a photocatalyst for the generation of H2 and O2 under visible-light irradiation for their research. Microwave radiation speed up the chemical reaction and decrease the energy consumed, consequently penetrating the reaction vessel and openly making available energy to the reactants and solvent with a great heat transfer rate. Microwave heating technique is unlike traditional techniques such as oil baths and heating chambers; this method is more effective and reliable. Microwave radiation, regarding to heat solvothermally pressurized and closed reaction system, the reactants can be reacted and transformed into products far more swiftly than using the conventional method. Dai and coworkers proposed a time-saving and economical process for the synthesis of g-C3N4 using microwave-assisted polymerization recently. Dai and coworkers then found out that the g-C3N4 sample achieved, showing submicrospheres and a high surface area of 90 m2 g−1, (Fig. 9) and was successfully synthesized at 180 °C under microwave irradiation condition for only 30 min which revealed an enhanced photocatalytic performance [71]. Experiments performed by Hu and coworkers also revealed that the microwave- synthesized g-C3N4 has good chemical and thermal stability and strong emission intensity than those of the conventional one [71]. Hu and coworkers also stated that microwave synthesized g-C3N4 performed better in visible-light-responsive photocatalysis.
Physical Vapor Deposition
It consists of magnetron sputtering, ion beam deposition (IBD), reaction sputtering, and pulsed laser deposition, and so forth. Reaction sputtering is the elementary method for preparation of composites. When this technique is used to prepare g-C3N4, the mass fraction of nitrogen is usually less than 40%. Conversely, to form 훽-C3N4, the system should consist of an adequate amount of nitrogen and stoichiometric ratio should reach 57%. Niu and his group [72] achieved the g-C3N4 on silicon substrate by using pulse laser evaporation C target, auxiliary deposition of atom nitrogen. Niu et al. studies found that the amount of N reached 40% in the films and then C, N atoms combined with nonpolar covalent bond. Successively, Sharma et al. [73] and Zhang et al. [74] also did some critical studies and then obtained CN푥 films by a similar method as discussed. Mihailescu and coworkers [75] also used ammonia instead of N2-manufactured hard CN푥 films with carbon nitrogen single bond, double bond, and triple bond and then found out that its optical band gap is 4.5 eV. From the recent study, what scientists frequently get are mixture films which comprise several crystal phases.
To consider the efficacy of prepared g-C3N4, photocatalytic hydrogen evolution using crystalline carbon nitrides (CNs) was proposed by Takanabe and his group [76]. Takanabe et al. acquired carbon nitrides by supramolecular aggregation (Table 3) which was further monitored by (Table 3) ionic melt polycondensation (IMP) using melamine and 2, 4, 6-triaminopyrimidine as a dopant. There are other few methods similar to what Takanabe and his group used in their experiment, see Table 3.
Applications of Graphitic Carbon Nitride
There are several emerging applications of this graphitic carbon nitride and such applications include based sensing, biomedical applications, wastewater and environmental treatment, solar energy utilization and being used in device making.
Solar energy Utilization
To increase the visible responsive activity of carbon nitride is not only dependent on controlling the molecule structures, synthesis, and preparation techniques of CN but also dependent on the ability to alter the electronic structures of these materials. Usually, under visible-light irradiations, carbon nitrides can be used to produce photoelectrode and thereby generating photocurrent. This ability of g-C3N4 is due to the exceptional reversible protonation and deprotonation nature. One of the greatest approaches is the use solar fuel from CO2 and water (produced by most photocatalysts) to produce H2, hydrocarbons, and syngas for energy and others [77, 78]. It was proposed that g-C3N4 has the potential of being metal-free and scalable photocatalysts for visible-light use based on the structure, synthesis, and preparation technique applied. A recent work by Liu and team [79] has suggested a novel development of sacrificial templating method for formulating mesoporous g-C3N4 spheres and a high-throughput scheme. This proposed technique can be used to synthesize g-C3N4 rods, and this is best for NADH regeneration (Fig. 10a–c) for successful production of energy and others.
Schematic drawing illustrating synthetic route (templating method) and the mechanism of charge separation and photocatalytic process over C3N4 and Ag@C3N4 photocatalysts under light irradiation. Reproduced with permission [124]. Copyright 2014 Elsevier.
Wastewater and Environmental Treatment
Most petrochemical, petrochemical, textile, and food industrial processes lead to pollution in the environment, to be precise, water bodies [80]. In the production of textiles, photographic materials, and printing materials, organic dyes are used and these dyes leach into most aquatic environment during the dying process [81]. Despite the harmful impact of these dyes on human and animal health, their biological and chemical degradation is challenging [82, 83]. Due this threat, there is a need to develop a superior oxidation process for the treatment of contaminated drinking water and non-degradable materials [84, 85]. Most researches [86,87,88,89,90] have proven that the use of semiconductors such as g-C3N4 for photocatalysis is the best method for the treatment of wastewater and environment due to their less harmful nature [86,87,88,89,90]. g-C3N4 is best known to be the potential photocatalysts for the degradation of numerous pollutants [16, 90, 91], with photophysical potentials of the parent nitride altered through doping with heteroatoms, heterojunction formation with other materials, and textural enhancements to expand surface area and porosity. The structure, synthesis, and preparation techniques of g-C3N4 nanosheets also determine the efficiency of the photocatalyst and its application in relation to wastewater treatment. Ultrathin g-C3N4 nanosheets derived from bulk g-C3N4 by exfoliation in methanol reveal heightened photocatalytic activity (Fig. 11) for methylene blue (MB) degradation [92]. Synthesizing and preparing of the candidate by doping metals such as Cu and Fe [93,94,95, 96] and non-metals such as B, C, O, or S [97,98,99,100], and co-doping [101,102,103] has been widely used by many scientists for water and environmental treatment. A promising solution to environmental depollution [104,105,106] is the combination of noble metals and g-C3N4 [107,108,109,110,111,112].
SEM images of (a) ST, (b) thermal condensation (TC), and (c) Microwave assisted synthesis (MW) samples; (d) magnification of MW sample; Photocatalytic degradation of MO solution over MW, ST, TC C3N4, and Ag-TiO2 samples irradiated under visible light. In the experiment, a blank test was performed in which the solution was irradiated without adding a catalyst. Reproduced with permission [125]. Copyright 2017 Elsevier
In summary, the unfeasible applications in wastewater and environmental pollution of most of the utmost well-versed photocatalysts is due to some of their demerit deterrents which includes, high cost, small scale, little photocatalytic activity, and thought-provoking recycle. Reasonably, in the area of environmental remediation, g-C3N4, TiO2-, and ZnO-based nano-material exhibit the most promising applications as result of their low cost, high photocatalytic activity, and no second pollution on the environment [3].
Biomedical and Sensing Applications
To increase the ability of g-C3N4 for sensing, biotherapy, and bioimaging usage, there is a need to alter the molecular structure, thereby enhancing the handling of the material in water. Due to the light photoluminenscence, highly recommended for biological related use, g-C3N4 nano-material is a very essential candidate for biomedical and sensing applications. The application of g-C3N4 for sensing, biotherapy, and bioimaging mainly considers its structure, synthesis, and preparative mechanisms. Zhang and coworkers [53] proposed that ultrathin g-C3N4 nanosheets could be used as biomarkers for the labeling of the cell’s membranes. g-C3N4 has also been suggested by Lin and co. to be a potential photosensitizers and pH-responsive drug nanocarriers for cancer imaging and therapy.
Future Perspectives
From the discussion, the future research of the g-C3N4 nano-based compound may focus on synthesizing innovative g-C3N4 nano-based particle which are responsive to morphology monitoring, evaluating the photocatalysis practicality and efficacy of traditional synthesis and preparative strategies of g-C3N4 nano-based compound, and then exploring the applications of diverse g-C3N4 nano-based particles in treating commercial wastewater, its effective application in solar energy utilization, environmental treatment, biomedical and sensing applications by fully assessing their photocatalytic ability, cost, energy consumption, and reusability.
Conclusions
In conclusion, this mini review climaxes the current advances on the structure and preparation techniques of full-bodied g-C3N4 nano-based material. Understandably, g-C3N4 has demonstrated to be one of the greatest favorable entrants suitable for scheming and assembling innovative composite photocatalysts. Thus, there is little uncertainty that the massive advancement of g-C3N4 nano-based particle will endure to develop in the near future. In view of that, more studies are also needed to making full use of the exceptional structural, synthesis, properties, and the preparation techniques of g-C3N4 nano-based particle.
Abbreviations
- g-C3N4 :
-
Graphite carbon nitride
- TiO2 :
-
Titanium oxide
- ZnO:
-
Zinc oxide
References
Fox M (1988) Photocatalytic oxidation of organic substances. In: Kluwer (ed) Photocatalysis and environment: trends and applications. New York Academic Publishers, New York, pp 445–467
Gaya UI, Abdullah AH (2008) Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: a review of fundamentals, progress and problems. J Photochem Photobiol C: Photochem Rev 9:1–12
Umar M, Aziz HA (2006) Photocatalytic degradation of organic pollutants in water. Org Pollutants: Monitory Risk Treatment 8:195–208 dx.doi.org. https://doi.org/10.5772/53699
Fujishima A, Honda K (1972) Electrochemical Photolysis of Water at a Semiconductor. Nature. 238:3489–3491
Boroski M, Rodrigues AC, Garcia JC, Sampaio LS, Nozaki J, Hioka N (2009) Combined electrocoagulation and TiO2 photoassisted treatment applied to wastewater effluents from pharmaceutical and cosmetic industries. J Hazard Mater 162:448–454
Wang X, Maeda K, Thomas A, Takanabe K, Xin G, Carlsson JM, Domen K, Antonietti M (2008) A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat Mater 8(76)
Zhang YJ, Thomas A, Antonietti M, Wang XC (2009) Activation of carbon nitride solids by protonation: Morphology changes, enhanced ionic conductivity, and photoconduction experiments. J Am Chem Soc 131:50–51
Li J, Shen B, Hong Z, Lin B, Gao B, Chen Y (2012) A facile approach to synthesize novel oxygen-doped g-C3N4 with superior visible-light photoreactivity. Chem Commun 48:12017–12019
Dai H, Gao X, Liu E, Yang Y, Hou W, Kang L, Fan J, Hu X (2013) Diam Relat Mater 38:109–117
Chang F, Xie YC, Li CL, Chen J, Luo JR, Hu XF, Shen JW (2013) A facile modification of g-C3N4 with enhanced photocatalytic activity for degradation of methylene blue. Appl Surf Sci. 280:967–974
Schneider J, Matsuoka M, Takeuchi M, Zhang J, Horiuchi Y, Anpo M, Bahnemann DW (2014) Understanding TiO2 photocatalysis: mechanisms and materials. Chem Rev 114:9919–9986
Nosaka Y, Nosaka AY (2017) Generation and detection of reactive oxygen species in photocatalysis. Chem Rev 117:11302–11336
Pei Z, Weng S, Liu P (2015) Enhanced Photocatalytic Activity by Bulk Trappingand Spatial Separation of Charge Carriers: A Case Study of Defect and Facet Mediated TiO 2 Graphical abstract Highlights. Elsevier B.V. https://doi.org/10.1016/j.apcatb.2015.06.045
Karamian E, Sharifnia S (2016) On the general mechanism of photocatalytic reduction of CO2. J CO2 Util 16:194–203
White JL, Baruch MF, Pander JE, Hu Y, Fortmeyer IC, Park JE, Zhang T, Liao K, Gu J, Yan Y et al (2015) Light-driven heterogeneous reduction of carbon dioxide: photocatalysts and photoelectrodes. Chem Rev 115:12888–12935
Kumar S, Karthikeyan S, Lee AF (2018) g-C3N4-based nanomaterials for visible light-driven photocatalysis. Catalysts 8(2):74
Tong H, Ouyang S, Bi Y, Umezawa N, Oshikiri M, Ye J (2012) Adv Mater 24:229–251
Kroke E, Schwarz M, Horath-Bordon E, Kroll P, Noll B, Norman AD (2002) Tri-s-triazine derivatives. Part i. From trichloro-tri-striazine to graphitic C3N4 structures. New J Chem 26(5):508–512
Liebig J (1834) Uber einige stickstoff-verbindungen. Eur J Organic Chem 10(1):1–47
Gmelin L (1835) Ueber einige Verbindungen des Melon’s. Eur J Organic Chem 15(3):252–258
Tian Y, Wang JZ, Yu WF, Cao R, Song Y, Ning X (2009) Effect of acetic acid on electrochemical deposition of carbon-nitride thin film. Sci China Ser E Technol Sci 52(6):1698–1702
Gu YS, Duan ZJ, Yi HG et al (1997) The preparation of crystalline β-C3N4 film. Physics 26(8):449–450
Li C, Cao CB, Zhu HS (2003) Electrodeposition of graphitelike carbon nitride thin films. J Synth Cryst 32(3):252–256
Yu KM, Cohen ML, Haller EE, Hansen WL, Liu AY, Wu IC (1994) Observation of crystalline C3N4. Phys Rev B 49(7):5034–5037
Thomas A, Fischer A, Goettmann F, Antonietti M, Mueller J-O, Schloegl R, Carlsson JM (2008) Graphitic carbon nitride materials: variation of structure and morphology and their use as metal free catalysts. J Mater Chem 18(41):4893–4908
Liu AY, Cohen ML (1989) Prediction of new low compressibility solids. Science 245(4920):841–842
Chen MY, Li D, Lin X, Dravid VP, Chung YW, Wong MS, Sproul WD (1993) Analytical electron-microscopy and raman-spectroscopy studies of carbon nitride thin-films. J Vac Sci Technol A 11(3):521–524
Teter DM, Hemley RJ (1996) Low-compressibility carbon nitrides. Science 271(5245):53–55
Zhang X, Gui Y, Dong X (2016) Preparation and application of TiO2 nanotube array gas sensor for SF6- insulated equipment detection: a review. Nanoscale Res Lett 11:302
Zhang X, Gui Y, Xiao H, Zhang Y (2016) Analysis of adsorption properties of typical partial discharge gases on Ni-SWCNTs using density functional theory. Appl Surf Sci 379:47–54
Song H, Zhang L, Yingying S, Yi L (2017) Recent advances ingraphitic carbon nitride-basedchemiluminescence, cataluminescence and electrochemiluminescence. J Analysis Testing 1(4):274–290
Wang EG, Chen Y, Guo LP (1997) Preparation and structure of C3N4- specimens on Ni substrate, science in China A. 27(2):154–157
Vinu A, Ariga K, Mori T, Nakanishi T, Hishita S, Golberg D, Bando Y (2005) Preparation and Characterization of Well-Ordered Hexagonal Mesoporous Carbon Nitride. Adv Mater 17:1648–1652 https://doi.org/10.1002/adma.200401643
Liang C, Hong K, Guiochon GA, Mays JW, Dai S (2004) Synthesis of a large-scale highly ordered porous carbon film by self-assembly of block copolymers. Angew Chem 43(43):5785–5789 https://doi.org/10.1002/anie.200461051
Groenewolt M, Antonietti M (2005) Synthesis of g-C3N4 Nanoparticles in Mesoporous Silica Host Matrices. Adv Mater 17:1789–1792 https://doi.org/10.1002/adma.200401756
Gago R, Jimenez I, Caceres D, Agullo-Rueda F, Sajavaara T, Albella JM, Climent-Font A, Vergara I, Raisanen J, Rauhala E (2001) Hardening mechanisms in graphitic carbon nitride films grown with N2/Ar ion assistance. Chem Mater 13(1):129–135
Wang Y, Wang F, Zuo Y, Zhang X, Cui LF (2014) Simple synthesis of ordered cubic mesoporous graphitic carbon nitride by chemical vapor deposition method using melamine. Mater Lett 136:271–273
Lu D, Fang P, Wu W, Ding J, Jiang L, Zhao X, Li C, Yang M, Li Y, Wang D (2017) Solvothermal-assisted synthesis of self-assembling TiO2 nanorods on large graphitic carbon nitride sheets with their anti-recombination in the photocatalytic removal of Cr (VI) and rhodamine B under visible light irradiation. Nanoscale 9(9):3231–3245
Yu Q, Guo S, Li X, Zhang M (2014) One-step fabrication and high photocatalytic activity of porous graphitic carbon nitride/graphene oxide hybrid by direct polymerization of cyanamide without templates. Russ J Phys Chem A 88(10):1643–1649
Yu Q, Li X, Zhang M (2014) One-step fabrication and high photocatalytic activity of porous graphitic carbon nitride synthesized via direct polymerisation of dicyandiamide without templates. Micro Nano Lett 9(1):1–5
Li X, Zhang J, Shen L, Ma Y, Lei W, Cui Q, Zou G (2009) Preparation and characterization of graphitic carbon nitride through pyrolysis of melamine. Appl Phys a-Mater Sci Process 94(2):387–392
Liu J, Zhang T, Wang Z, Dawson G, Chen W (2011) Simple pyrolysis of urea into graphitic carbon nitride with recyclable adsorption and photocatalytic activity. J Mater Chem 21(38):14398–14401
Dong F, Sun Y, Wu L, Fu M, Wu Z (2012) Facile transformation of low cost thiourea into nitrogen-rich graphitic carbon nitride nanocatalyst with high visible light photocatalytic performance. Catal Sci Technol 2(7):1332–1335
Huang Z, Li F, Chen B, Yuan G (2014) Nanosheets of graphitic carbon nitride as metal-free environmental photocatalysts. Catal Sci Technol 4(12):4258–4264
Long B, Lin J, Wang X (2014) Thermally-induced desulfurization and conversion of guanidine thiocyanate into graphitic carbon nitride catalysts for hydrogen photosynthesis. J Mater Chem A 2(9):2942–2951
Tian J, Liu Q, Asiri AM, Al-Youbi AO, Sun X (2013) Ultrathin graphitic carbon nitride nanosheet: a highly efficient fluoro-sensor for rapid, ultrasensitive detection of Cu2? Anal Chem 85(11):5595–5599
Tian J, Liu Q, Ge C, Xing Z, Asiri AM, Al-Youbi AO, Sun X (2013) Ultrathin graphitic carbon nitride nanosheets: a low-cost, green, and highly efficient electrocatalyst toward the reduction of hydrogen peroxide and its glucose biosensing application. Nanoscale 5(19):8921–8924
Tian J, Liu Q, Asiri AM, Alamry KA, Sun X (2014) Ultrathin graphitic C3N4 nanosheets/graphene composites: efficient organic electrocatalyst for oxygen evolution reaction. ChemSusChem 7(8):2125–2130
Yan H (2012) Soft-templating synthesis of mesoporous graphitic carbon nitride with enhanced photocatalytic H2 evolution under visible light. Chem Commun 48(28):3430–3432
Yang Z, Zhang Y, Schnepp Z (2015) Soft and hard templating of graphitic carbon nitride. J Mater Chem A 3(27):14081–14092
Xie RL, Zong ZM, Liu FJ, Wang YG, Yan HL, Wei ZH, Mayyas M, Wei X-Y (2015) Nitrogen-doped porous carbon foams prepared from mesophase pitch through graphitic carbon nitride nanosheet templates. RSC Adv 5(57):45718–45724
Song Z, Lin T, Lin L, Lin S, Fu F, Wang X, Guo L (2016) Invisible security ink based on water-soluble graphitic carbon nitride quantum dots. Angew Chemie-Int Ed 55(8):2773–2777
Zhan Y, Liu Z, Liu Q, Huang D, Wei Y, Hu Y, Lian X, Hu C (2017) A facile and one-pot synthesis of fluorescent graphitic carbon nitride quantum dots for bio-imaging applications. New J Chem 41(10):3930–3938
Li H, Shao FQ, Huang H, Feng JJ, Wang AJ (2016) Eco-friendly and rapid microwave synthesis of green fluorescent graphitic carbon nitride quantum dots for vitro bioimaging. Sens Actuators B Chem 226:506–511
Barman S, Sadhukhan M (2012) Facile bulk production of highly blue fluorescent graphitic carbon nitride quantum dots and their application as highly selective and sensitive sensors for the detection of mercuric and iodide ions in aqueous media. J Mater Chem 22(41):21832–21837
Zhou Z, Shang Q, Shen Y, Zhang L, Zhang Y, Lv Y, Li Y, Liu S, Zhang Y (2016) Chemically modulated carbon nitride nanosheets for highly selective electrochemiluminescent detection of multiple metal-ions. Anal Chem 88:6004–6010
Ma ZB (2006) Progress in the synthesis and characterization of carbon nitride crystals. New Carbon Mater 21(3):276–283
Liang XR, Jiang YL, Kong LY et al (2013) The synthesis, application and research progress of carbon nitride (C3N4) materials. New Technol New Process 1:88–90
Gu Y, Chen L, Qian Y, Zhang W, Ma J (2005) Synthesis of nanocrystalline boron carbide via a solvothermal reduction of CCl4 in the presence of amorphous boron powder. J Am Ceram Soc 88:225–227
Dante RC, Martin-Ramos P, Correa-Guimaraes A, Martin-Gil J (2011) Synthesis of graphitic carbon nitride by reaction of melamine and uric acid. Mater Chem Phys 130:1094–1102
Cheetham AK, Férey G, Loiseau T (1999) Open-framework inorganic materials. Angew Chem Int Ed 38:3268–3292
Feng S, Xu R (2001) New materials in hydrothermal synthesis. Acc Chem Res 34:239–247
Xiang Q, Yu J, Jaroniec M (2011) Preparation and enhanced visible-light photocatalytic H2-production activity of graphene/C3N4 composites. J Phys Chem C 115:7355–7363 https://doi.org/10.1021/jp200953k
Niu W, Zhang L, Xu G (2010) Shape-controlled synthesis of single-crystalline palladium nanocrystals. ACS Nano 4:1987–1996
Loumagne F, Langlais F, Naslain R, Schamm S, Dorignac D, Sevely J (1995) Physicochemical properties of SiC-based ceramics deposited by low pressure chemical vapor deposition from CH3SiCl3H2. Thin Solid Films 254:75–82
Kelly JP, Kanakala R, Graeve OA (2010) A solvothermal approach for the preparation of nanostructured carbide and boride ultra-high-temperature ceramics. J Am Ceram Soc 93:3035–3038
Bointon TH, Barnes MD, Russo S, Craciun MF (2015) High quality monolayer graphene synthesized by resistive heating cold wall chemical vapor deposition. Adv Mater 27(28):4200–4206
Li X, Cai W, An J, Kim S, Nah J, Yang D et al (2009) Large-area synthesis of high-quality and uniform graphene films on copper foils. Science 324(5932):1312–1314
Laget V, Hornick C, Rabu P, Drillon M, Turek P, Ziessel R (1998) Multilayered ferromagnets based on hybrid organic–inorganic derivatives. Adv Mater 10:1024–1028
Agaskar PA (1989) Organo lithic macromolecular materials derived from vinyl- functionalized sphero silicates: novel potentially microporous solids. J Am Chem Soc 111(17):6858–6859
Hu C, Chu Y-C, Wang M-S, Xiao-Han W (2017) Rapid synthesis of g-C3N4 spheres using microwave-assisted solvothermal method for enhanced photocatalytic activity. J Photochem Photobiol A Chem 348:8–17
Niu C, Lu YZ, Lieber CM (1993) Experimental realization of the covalent solid carbon nitride. Science 261(5119):334–337
Sharma AK, Ayyub P, Multani MS (1996) Synthesis of crystalline carbon nitride thin films by laser processing at a liquid-solid interface. Appl Phys Lett 69:3489 https://doi.org/10.1063/1.117261
Zhang ZJ, Fan SS, Huang JL, Lieber CM (1996) Diamond like properties in a single phase carbon nitride solid. Appl Phys Lett 68(19):2639–2641
Mihailescu IN, Gyorgy E, Alexandrescu R et al (1998) Optical studies of carbon nitride thin films deposited by reactive pulsed laser ablation of a graphite target in low pressure ammonia. Thin Solid Films 323(1–2):72–78
Bhunia MK, Yamauchi K, Takanabe K (2014) Harvesting solar light with crystalline carbon nitrides for efficient photocatalytic hydrogen evolution. Angew Chem Int Ed 53:1–6
Styring S (2012) Artificial photosynthesis for solar fuels. Faraday Discuss 155:357–376
Gao Q, Sun S, Li X et al (2016) Enhancing performance of CdS quantum dot-sensitized solar cells by two-dimensional gC3N4 modified TiO2 nanorods [J]. Nanoscale Res Lett 11(1):463
Liu J, Cazelles R, Zhou H, Galarneau A, Antonietti M (2014) Phys Chem Chem Phys 16:14699–14705
Richardson SD, Ternes TA (2014) Water analysis: emerging contaminants and current issues. Anal Chem 86:2813–2848
Gao Q, Luo J, Wang X et al (2015) Novel hollow α-Fe2O3 nanofibers via electrospinning for dye adsorption[J]. Nanoscale Res Lett 10(1):176
Bolong N, Ismail AF, Salim MR, Matsuura T (2009) A review of the effects of emerging contaminants in wastewater and options for their removal. Desalination 239:229–246
Lofrano G, Meriç S, Zengin GE, Orhon D (2013) Chemical and biological treatment technologies for leather tannery chemicals and wastewaters: a review. Sci Total Environ 461–462:265–281
Karthikeyan S, Dionysiou DD, Lee AF, Suvitha S, Maharaja P, Wilson K, Sekaran G (2016) Hydroxyl radical generation by cactus-like copper oxide nanoporous carbon catalysts for microcystin-LR environmental remediation. Catal Sci Technol 6:530–544
Karthikeyan S, Pachamuthu MP, Isaacs MA, Kumar S, Lee AF, Sekaran G (2016) Cu and Fe oxides dispersed on SBA-15: a Fenton type bimetallic catalyst for N,N-diethyl-p-phenyl diamine degradation. Appl Catal B Environ 199:323–330
Binas V, Venieri D, Kotzias D, Kiriakidis G (2017) Modified TiO2 based photocatalysts for improved air and health quality. J Mater 3:3–16
Ollis DF, Pelizzetti E, Serpone N (1991) Photocatalyzed destruction of water contaminants. Environ Sci Technol 25:1522–1529
Hoffmann MR, Martin ST, Choi W, Bahnemann DW (1995) Environmental applications of semiconductor photocatalysis. Chem Rev 95:69–96
Jo W-K, Kumar S, Isaacs MA, Lee AF, Karthikeyan S (2017) Cobalt promoted TiO2/GO for the photocatalytic degradation of oxytetracycline and Congo Red. Appl Catal B Environ 201:159–168
Ong W-J, Tan L-L, Ng YH, Yong S-T, Chai S-P (2016) Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: are we a step closer to achieving sustainability? Chem Rev 116:7159–7329
Dong G, Zhang Y, Pan Q, Qiu J (2014) A fantastic graphitic carbon nitride (g-C3N4) material: electronic structure. photocatalytic and photoelectronic properties. J Photochem Photobiol C Photochem Rev 20:33–50
Pan C, Xu J, Wang Y, Li D, Zhu Y (2012) Dramatic activity of C3N4/BiPO4 photocatalyst with core/shell structure formed by self-assembly. Adv Funct Mater 22:1518–1524
Li Z, Kong C, Lu G (2016) Visible photocatalytic water splitting and photocatalytic two-electron oxygen formation over Cu- and Fe-doped g-C3N4. J Phys Chem C 120:56–63
Tonda S, Kumar S, Kandula S, Shanker V (2014) Fe-doped and -mediated graphitic carbon nitride nanosheets for enhanced photocatalytic performance under natural sunlight. J Mater Chem A 2:6772–6780
Gao J, Wang J, Qian X, Dong Y, Xu H, Song R, Yan C, Zhu H, Zhong Q, Qian G et al (2015) One-pot synthesis of copper-doped graphitic carbon nitride nanosheet by heating Cu–melamine supramolecular network and its enhanced visible-light-driven photocatalysis. J Solid State Chem 228:60–64
Kong JZ, Zhai HF, Zhang W et al (2017) Visible light-driven photocatalytic performance of N-doped ZnO/gC 3 N 4 nanocomposites. Nanoscale Res Lett 12(1):526
Yan SC, Li ZS, Zou ZG (2010) Photodegradation of rhodamine B and methyl Orange over boron-doped g-C3N4 under visible light irradiation. Langmuir 26:3894–3901
Liu G, Niu P, Sun C, Smith SC, Chen Z, Lu GQ, Cheng H-M (2010) Unique electronic structure induced high photoreactivity of sulfur-doped graphitic C3N4. J Am Chem Soc 132:11642–11648
Ma X, Lv Y, Xu J, Liu Y, Zhang R, Zhu Y (2012) A strategy of enhancing the photoactivity of g-C3N4 via doping of nonmetal elements: a first-principles study. J Phys Chem C 116:23485–23493
Panneri S, Ganguly P, Mohan M, Nair BN, Mohamed AAP, Warrier KG, Hareesh US (2017) Photoregenerable, bifunctional granules of carbon-doped g-C3N4 as adsorptive photocatalyst for the efficient removal of tetracycline antibiotic. ACS Sustain Chem Eng 5:1610–1618
Hu S, Ma L, You J, Li F, Fan Z, Lu G, Liu D, Gui J (2014) Enhanced visible light photocatalytic performance of g-C3N4 photocatalysts co-doped with iron and phosphorus. Appl Surf Sci 311:164–171
Oh W-D, Lok L-W, Veksha A, Giannis A, Lim T-T (2018) Enhanced photocatalytic degradation of bisphenol A with Ag-decorated S-doped g-C3N4 under solar irradiation: performance and mechanistic studies. Chem Eng J 333:739–749
Hu S, Ma L, Xie Y, Li F, Fan Z, Wang F, Wang Q, Wang Y, Kang X, Wu G (2015) Hydrothermal synthesis of oxygen functionalized S-P codoped g-C3N4 nanorods with outstanding visible light activity under anoxic conditions. Dalton Trans 44:20889–20897
Papageorgiou DG, Kinloch IA, Young RJ (2017) Mechanical properties of graphene and graphene-based nanocomposites. Progress in Materials Science 90:75–127
Fu J, Yu J, Jiang C, Cheng B (2017) g-C3N4-based heterostructured photocatalysts. Adv Energy Mater.
Mamba G, Mishra AK Graphitic carbon nitride (g- C3N4) nanocomposites: A new and exciting generation of visible light driven photocatalysts for environmental pollution remediation. Applied Catalysis B, Environmental. https://doi.org/10.1016/j.apcatb.2016.05.052
Li K, Zeng Z, Yan L, Luo S, Luo X, Huo M, Guo Y (2015) Fabrication of platinum-deposited carbon nitride nanotubes by a one-step solvothermal treatment strategy and their efficient visible-light photocatalytic activity. Appl Catal B Environ 165:428–437
Zhou X, Zhang G, Shao C, Li X, Jiang X, Liu Y (2017) Fabrication of g-C3N4/SiO2-Au composite nanofibers with enhanced visible photocatalytic activity. Ceram Int 43:15699–15707
Tonda S, Kumar S, Shanker V (2016) Surface plasmon resonance-induced photocatalysis by Au nanoparticles decorated mesoporous g-C3N4 nanosheets under direct sunlight irradiation. Mater Res Bull 75:51–58
Patnaik S, Sahoo DP, Parida K (2018) An overview on Ag modified g-C3N4 based nanostructured materials for energy and environmental applications. Renew Sust Energ Rev 82:1297–1312
Ling Y, Liao G, Feng W, Liu Y, Li L (2017) Excellent performance of ordered Ag-g-C3N4/SBA-15 for photocatalytic ozonation of oxalic acid under simulated solar light irradiation. J Photochem Photobiol A Chem 349:108–114
Fu Y, Liang W, Guo J, Tang H, Liu S (2018) MoS2 quantum dots decorated g-C3N4/Ag heterostructures for enhanced visible light photocatalytic activity. Appl Surf Sci 430:234–242
Ke Wang, Qin Li, Baoshun Liu, Bei Cheng, Wingkei Ho, Jiaguo Yu. Sulfur-doped g-C3N4 with enhanced photocatalytic CO2-reduction performance. Applied Catalysis B: Environmental 176–177 (2015) 44–52
Chu Y-C, Wang M-S, Hu C, Wu X-H (2017) Rapid synthesis of g-C3N4 spheres using microwave-assisted solvothermal method for enhanced photocatalytic activity. Journal of Photochemistry and Photobiology A: Chemistry 348:8–17
Adormaa BB, Darkwah WK, Ao Y (2018) Oxygen vacancies of the TiO2 nano-based composite photocatalysts in visible light responsive photocatalysis. RSC Adv. 8:33551
Bai X, Zong R, Li C, Liu D, Liu Y, Zhu Y (2014) Enhancement of visible photocatalytic activity via Ag@C3N4core-shell plasmonic composite. Applied Catalysis B: Environmental 147:82–91
Ding F, Yang D, Tong Z, Nan Y, Wang Y, Zouad X, Jiang Z (2017) Graphitic carbon nitride-based nanocomposites as visible-light driven photocatalysts for environmental purification. Environ Sci: Nano 4:1455
Song L, Pang Y, Zheng Y, Ge L (2017) Hydrothermal synthesis of novel g-C3N4/BiOCl heterostructure nanodiscs for efficient visible light photodegradation of Rhodamine B. Appl. Phys. A 123:500. https://doi.org/10.1007/s00339-017-1091-2
Goettmann F, Fischer A, Antonietti M, Thomas A (2006) Chemical synthesis of mesoporous carbon nitrides using hard templates and their use as a metal-free catalyst for Friedel–Crafts reaction of benzene. Angew Chem Int Ed 45:4467–4471
Ye S, Wang R, Ming-Zai W, Yuan。 Y-P (2015) A review on g-C3N4 for photocatalytic water splitting and CO2 reduction。Applied. Surface Science 358:15–27
Gholipour MR, Dinh C-T, Béland F, Do T-O (2015) Nanocomposite heterojunctions as sunlight-driven photocatalysts for hydrogen production from water splitting. Nanoscale 7:8187–8208
Lakhi KS, Park D-H, Al-Bahily K, Cha W, Viswanathan B, Choy J-H, Vinu A Mesoporous carbon nitrides: synthesis, functionalization, and applications. Chem Soc Rev. https://doi.org/10.1039/c6cs00532b
Meng Y, Gu D, Zhang F, Shi Y, Cheng L, Feng D, Wu Z, Chen Z, Wan Y (2006) A Family of Highly Ordered Mesoporous Polymer Resin and Carbon Structures from Organic−Organic Self-Assembly. Stein A, Zhao D Chem Mater 18:4447–4464 https://doi.org/10.1021/cm060921u
Wang Y, Wang X, Antonietti M, Zhang Y (2010) Facile one-pot synthesis of nanoporous carbon nitride solids by using soft templates. Chem Sus Chem 3(4):435–439 https://doi.org/10.1002/cssc.200900284.67.Y
Wang Y, Zhang J, Wang X, Antonietti M, Li H (2010) Boron- and Fluorine-Containing Mesoporous Carbon Nitride Polymers: Metal-Free Catalysts for Cyclohexane Oxidation, Angew. Chem Int Ed 49:3356–3359
Acknowledgements
Williams Kweku Darkwah was the recipient of a scholarship from the China Scholarship Council (CSC) for the duration of this work.
Funding
This work was financially supported by grants from the National Science Funds for Creative Research Groups of China (No. 51421006), the Natural Science Foundation of China (51679063), the Key Program of National Natural Science Foundation of China (No. 41430751), the National Key Plan for Research and Development of China (2016YFC0502203), Fundamental Research Funds (No. 2016B43814), and PAPD.
Author information
Authors and Affiliations
Contributions
YA conceived the study and supervised the whole study. WKD drafted the manuscript including the design of the figures. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Authors’ Information
WKD is a Master’s degree student (supervised by Prof. Yanhui Ao) at Environmental Engineering Department, College of Environment, Hohai University, Nanjing, China. He received his BSc degree in University of Cape Coast, Cape Coast, Ghana. His research interest mainly focusses on photocatalysis based water remediation technology using nano-materials.
YA is working as a Professor in Hohai University, Nanjing, China. He received his Doctorate degree in Southeast University, Nanjing, China. He has published more 130 academic papers and also has more than 7 patents. His research interests mainly focus on new photocatalysis-based water remediation technology using nano-materials, water resources protection, behavior of manufactured nano-materials in environment, and environmental friendly materials.
Ethics Approval and Consent to Participate
Not applicable
Consent for Publication
Not applicable
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Darkwah, W.K., Ao, Y. Mini Review on the Structure and Properties (Photocatalysis), and Preparation Techniques of Graphitic Carbon Nitride Nano-Based Particle, and Its Applications. Nanoscale Res Lett 13, 388 (2018). https://doi.org/10.1186/s11671-018-2702-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11671-018-2702-3