Abstract
This review article summarizes the development of different kinds of materials that evolved interest in all field of science particularly on new nano-materials which possess both electric and magnetic properties at the nanoscale. Materials of such kind possessing both magnetic and electric properties have tremendous applications and own an intensive research activity. These materials induce new properties which are particularly important in electronic and magnetic devices and even in the materials where magnetic property will change by electric field or vice versa. The discovery of such ferroic properties for scientific applications is the need of hour and spreads an exciting new area that has technical and commercial potential for the discovery of advanced materials. In recent studies, the actual path by which the multiferroic properties exist has been focused and new metal oxide compounds were discovered. The understanding of the structure of these compounds through research describes a wide range of applications and the challenges of these multiferroic materials that need to be explored. In this study, fundamental aspects and structural variations of ternary transition metal oxides have been covered which possess novel properties in storage devices such as hard disk platters and magnetic read heads.
Similar content being viewed by others
Introduction
Magnetic properties of objects at nanoscale range have been given the name of concept nanomagnetism with a prone area of research in all scientific fields. The properties and applications of magnetic nanoparticles, nanofilms, nanorods, and many more have been used earlier also in geology as ferrofluids and have enough scope to explore in the future [1]. These advanced materials have been used in other aspects, such as in loudspeakers and in the medical field for drug delivery [2] or even in magnetic hyperthermia [3]. The storage materials at very small size have usually found good efficiency if fabricated in small devices that reduces the dimension of machines. These small devices made up of magnetic nanoparticles play an important role in industries and most importantly in biomedical applications [4]. These materials have been applied to magnetic resonance imaging (MRI) devices that enable and visualize the local environment of tissue cells of cancer cells or tumors [5]. These magnetic nanoparticles have unique biomedical applications particularly to treat central nervous diseases and need to explore further to find innovative approaches in drug delivery to treat Central Nervous System (CNS) diseases [6].
Spontaneous magnetization can be created in a loop-like structure called hysteresis by the applied magnetic field. This particular feature of materials has given the name of ferromagnetic materials, and this property of materials originates from the electron spins and their orbital motion around the nucleus. In the absence of an external magnetic field, the magnetic moments are randomly oriented but when a field is applied, these spins are locked into a particular order and small group of spins to form domain-like structures. The structures and the typical hysteresis loop of these magnetic materials are shown in Fig. 1. Transition metals like nickel, cobalt, chromium, and iron have magnetic moments originating from spin orientations and also have an orbital contribution to the magnetic field [7]. These interactions among the spins aligned in one particular order at a certain temperature below the Curie temperature (Tc) and above this temperature ferromagnetic domains overcome thermal energy [8]. The very unique characteristic of ferromagnetic property is to have hysteresis loop, featured by the existence of saturation magnetization (Ms) above which there is no increase of further magnetic property whatsoever be the magnitude of applied magnetic field. Another feature of ferromagnetic materials, remanent magnetization (Mr), stores even in the absence of applied magnetic field, and this property is related with the memory or storage capacity of materials. Further, these ferromagnetic materials are specified with the coercive field (Hc) which measures the magnitude of reverse direction of the magnetic field to remove all its magnetization effect. These three properties are of prime importance in finding out the potential phase of ferromagnetic material. There is a competition between exchange magnetostatic and anisotropy energies, and there exist the long- and short-order interaction domains [9].
Ferroelectric property [10] characterized by the existence of polarization in the presence of applied electric field is analogous with the ferromagnetic property. The difference between the ferroelectric and ferromagnetic lies in the structure of materials but not with atoms, so ferroelectric is an intrinsic property. This property depends on the whole structure and symmetry of compounds and the order, disorder, and displacement of ions that gives rise to the mechanism of ferroelectricity [11,12,13]. Structured polarization is related with the ferroelectric property that results in the hysteresis loop formed from electric domains. There is a certain temperature below which the phase change from paraelectric to ferroelectric called transition temperature, that in turn depends on the nature of materials. These mini domain characteristics of hysteresis are shown in Fig. 2 and in some manner match with the magnetic hysteresis loop. By plotting a graph between electric polarization versus applied electric field, a loop-like structure was formed with saturation polarization (Ps), remanent polarization (Pr). and coercive field (Hc) [14]. Here, the domain starts to align in positive field direction that gives rise to rapid polarization and reaches to maximum polarization called saturation polarization, and beyond this, there is no further increase in the value of polarization. Further, if the applied field is reversed, polarization tends to decrease and reaches to a particular value where the applied field is zero. Remanent polarization (residual polarization in the material when the electric field is totally removed) is the measure of retaintivity or remanence of the materials used specifically for memory and storage capacity. In order to attain zero polarization, the applied electric field must be further decreased. The magnitude of the applied electric field where the whole polarization becomes zero is called the coercive field. These values are characteristics of hysteresis that depends on the structure, nature, and size of ferroelectric materials [15].
Multiferroic: a Unique and Novel Property [16]
The concept of multiferroic was introduced by H. Schmidt in 1994 [17], and as per the latest definition, multiferroic materials possess simultaneous two or more than two ferroic phases together in a single material [18]. These materials became subject of research to investigate the chemical nature and to study the solid state physics [19]. Bulk research in this field helped to develop a lot of new ideas to utilize in device applications. One of the ideas is to introduce the multiferroic bits that may store information in the form of magnetization and polarization. There are only few materials which have two or more than two ferroic properties and hence the multiferroic materials are rare [20]. This trend of materials having one or more than two properties has been shown in Fig. 3, where it clearly indicates that there are very few materials which show the multiferroic behavior [21]. This is the reason why this field of research is a challenge for the present world and needs to be focussed [22]. Rare existence of multiferroics is related with the mechanism of ferroelectric behavior which demands empty d orbitals, and on the other side, ferromagnetism needs partially filled d orbitals [23, 24]. In order to compensate this sort of controversy and to achieve the multiferroic nature, the structure of the materials needs to be tuned in such a way that an atom may move from the center to form electric dipoles and should be related with magnetic moments. This will lead to either an alternative mechanism for magnetism or ferroelectricity. There are still certain things which may be explored at the nanoscale. The multiferroic nature of nanostructured materials may open new horizons in the applications of making small efficient devices like computer chips, and many more. Recent research is focusing on nano-multiferroic materials for fabrication, design, and applications. The ferroeclectric domain wall structures and the position of magnetic ions plays an important role to get the new functionility for the development of novel devices. The formation, engineering, and application by changing the structures can be used to carry the information in the latest devices. Continuous interest and growing space have been given to multiferroic materials that resulted in the fourth ferroic order called ferrotoroidicity [25, 26] and also determined the electrical conductivity domain walls that are different from bulk materials related with memory properties [27]. Quite a new interesting thing was also observed with the help of film deposition techniques, that the electric field gives the magnetism at room temperature [28]. Although, the multiferroic study has achieved appreciable interest from all the researchers around the world, there is still a poor approach of commercializing the multiferroic materials which need to be accelerated in the near future.
General classification of multiferroic materials. Adapted from Eerenstein et al. [21]
Various Classes of Multiferroic Compounds on the Basis of Structure
Bismuth Ferrites (BiFeO3 Compounds)
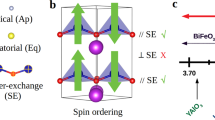
Bismuth ferrite ternary oxides and the derivative compounds are perovskite structures and are promising multiferroic compounds [29]. This ABO3 perovskite bismuth ferrite compound has ferroelectricity from the lone pair of electrons at central metal A (Bi3+) ion that distorts from the position and the symmetry of the compound lost which provides the ferroelectric property [30]. The cation at the site of B position is Fe3+ ion which is small and has unpaired d electrons that give the magnetic properties of BiFeO3 compound as shown in Fig. 4 [31]. Here, it can be concluded that polarization is caused by Bi3+ lone pair electrons present in 6s2 orbitals and magnetic property arises from Fe3+ ions. The fabrication of BiFeO3 nano-compound may lead to a new direction of research that will help to build interesting multiferroic materials. There were issues of leakage current that reduced the electrical parameters of bismuth ferrites and was later improved by the addition of strontium-zirconium ions into the BiFeO3-BaTiO3 composites. Further, phase structure, surface texture, and electrical properties were also studied systematically [32]. Much research was carried out in ferroelectric perovskite BiFeO3 for many application purposes, but has rarely been investigated for the energy conversion of tiny mechanical motions in electricity in spite of its large theoretical remnant polarization. But there was one report which showed that BiFeO3 nanomaterials have such a potential for large-scale lead-free piezoelectric nanogenerator and these nanoparticles were synthesized by a sol-gel process [33]. Bi5Ti3FeO15 (BTF) multiferroic lead-free nanofibers were fabricated by electrospinning and exhibit an effective micro-piezoelectric coefficient with benign micro-ferroelectricity [34]. Further, the coupling behavior between macro-ferroelectric and magnetoelectric was found by non-sintering and pressing for the first time and is smaller than Bi5Ti3FeO15 ceramic. The magnetic moments of BiFeO3 were balanced each other by two Fe ions spinning in the opposite direction within the cell, and the band gap was found around 20.5 eV [35]. Density of states were analyzed that indicates that the valence band consists of Fe-d and O-p states, while the conduction band is composed of Fe-d and Bi-p states. The dielectric function, absorption, refractive index, extinction coefficient, reflectivity, and electron energy loss were also reported for BiFeO3.
Yttrium Magnetite (YMnO3) Compounds
It seems that YMnO3 compound has the same perovskite ABO3 type structure, but it has a different crystal structure and electronic arrangements. In contrast to the conventional perovskites, hexagonal manganites have their Mn3+ ions with 5-fold coordination, located at the center of an MnO5 trigonal bi-prism. R ions, on the other hand, have 7-fold coordination unlike the cubic coordination in perovskites. The layer of Y3+ ions differentiates the two-dimensional MnO5 biprism as shown in Fig. 5, which represents the YMnO3 unit cell showing ionic structures. A new concept of antiferromagnetic ferroelectricity was found in YMnO3, and the geometric structure leads the ferroelectric properties which couples with the magnetic property of YMnO3 compound [36]. The tilting of MnO5 trigonal biprism results in the loss of inversion symmetry in the structure that leads out the ferroelectric properties of YMnO3-type compounds [37]. The coupling between the ferroelectricity and magnetic order is quite unlike, and this is the main reason why magnetoelectric coupling could not be possible in such type of materials. But the ion movements in the tilting-layered MnO5 polyhedra lead to the net polarization effect [38, 39] as shown in Fig. 6. It was also reported that hexagonal YMnO3 nanofibers prepared by the sol-gel method and the prepared spun fibers were dried at 125 °C with uniform diameter [40]. In an increase in temperature of the prepared sample, there was an adequate change in morphology and diameter range with homogenous chemical constituents over its length.
Crystal structure of YMnO3 featuring layers of MnO5 polyhedra and Y atom in between the layers. Adapted from Wadati et al. [38]
Three-dimensional schematic view of YMnO3 in the polarized states. Adapted from Spaldin et al. [39]
Rare Earth (RMO3, M = Fe, Cr, Mn ) Multiferroic Compounds
The latest research found that rare earth metal ternary oxides that may contain iron, manganese, and chromium elements at the B site show multiferroic properties in which weak ferromagnetic is accompanied by the room temperature ferroelectric behavior [41]. In case of RFeO3 compounds, the structure of such type of compounds is orthorhombic unit cells [42] with distorted perovskite structure. This distortion is just because of rare earth ion R3+ positions and the presence of Fe3+ ions in an octahedral environment. Such structures have FeO6 octahedra in the three dimension, one of the O2- ions forms one common apex between the two octahedra, and the two iron atoms provide the superexchange bond through O2- ions. In this concept, the Fe atoms are slightly canted that results in the weak ferromagnetic interactions [43]. Since the RFeO3 compounds are included in the family of centrosymmetric ferrites, there still exists the room temperature ferroelectric property. This unusual behavior can be explained with the literature which reported a SmFeO3 compound where the non-equivalent spins are responsible for the induced ferroelectric property and were given the name of antiferromagnetic ordering-induced ferroelectricity [44] which has been shown in Fig. 7.
Crystal structure and magnetic spectra of orthorhombic SmFeO3. Adapted from Scoot et al. [44]
The second class of rare earth multiferroic oxides is chromium-based RCrO3 compounds. In place of FeO6 structure, antiphase tilting of CrO6 octahedra as shown in Fig. 8 was present in orthorhombic (RCrO3, R = Y, Gd, Tb) multiferroic compounds. The polarization of ferroic nature couples with the magnetic ordering of Cr ion sublattices, and the well-known interaction Dzyaloshinskii-Moriya (DM) gives rise to the weak ferromagnetic properties of Cr3+ ions [45]. GdCrO3 compounds, the magnetic moment of Cr ions, are antiparallel to its nearest cations and are represented by G-type configuration. The class of ferroelectricity of RCrO3 compounds is still not explained properly, while it was assumed that off-centring distortion has been proposed for the origin of ferroelectric behavior. This kind of mechanism was reported in bulk, nano, thin films of RCrO3 compounds [46,47,48]. In the presence of applied magnetic field, the strength of polarization can be varied in case of GdCrO3 compounds. YCrO3 is orthorhombic but still is ferroelectric as the Cr atoms are displaced from the position in a particular direction which results in the polarization. This shows the new concept that can be visualized by many unusual properties of multi-functionalized materials.
Distorted orthorhombic perovskite crystal structure of RCrO3. Adapted from Fender et al. [45]
Cubic GdFeO3 particle by a simple hydrothermal synthesis route and its photoluminescence and magnetic properties were investigated [49]. Through the investigation of the photoluminescence and magnetic properties, the orthorhombic cubic GdFeO3 particles exhibited very good doped luminescence, which emits different colored light when doped with different rare earth elements. The GdFeO3 particles contain paramagnetic properties. It could be an excellent luminescence and magnetic material. High magnetoelectric coupling by using one single crystal of DyFeO3 and GdFeO3 has been reported before, but the multiferroic nature occurs only at very low temperature [50]. Solid-state powder synthesis of GdFeO3 and GdCrO3 involves the extensive mechanical grinding of the required oxides (Gd2O3, Fe2O3, and Cr2O3) at high enough calcination temperature ∼ 1800 °C. A simple sonochemical method for the synthesis of nanoparticles of a series of rare earth orthoferrites was reported. This sonochemical process is enabling the synthesis of nanoparticles of the rare earth orthoferrites at a substantially lower calcination temperature by using simple precursors, iron pentacarbonyl, and rare earth carbonates. It is particularly noteworthy that the cogeneration of the garnet phase has not been observed, as is usual with the conventional methods. The drastic reduction in the calcination temperature could be due to the ultrasonic generation of amorphous iron oxide from Fe(CO)5. Nanosized GdFeO3, ErFeO3, TbFeO3, and EuFeO3 were prepared by this method, and their magnetic properties were also studied in detail [51]. Highly crystalline orthoferrite nanoparticles (type La1−x GdxFeO3, where x = 0 to 1) were prepared using the self-combustion method. Our particular interest is in the characterization of the structural and magnetic properties of given compounds with a strong emphasis on the role of Gd3+ ions in the modulation of the structure and magnetic response [52]. Perovskites with composition MFeO3 are a class of materials having potential applications such as catalysts [53], sensors, [54] semiconductors, and [55] magnetic and magneto-optical materials [56]. The phase-selective synthesis of LnFeO3 (Ln = rare earth) is challenging because there are undesired coexisting phases [57, 58]. Visible-light-driven Gd2Ti2O7/GdCrO3 composite for hydrogen evolution has been reported, and a series of Gd2Ti2O7/GdCrO3 composites are prepared by solid-state combustion. The photocatalytic activity of the composites is examined towards hydrogen production without using any co-catalyst under visible light illumination. The rate of formation of hydrogen is measured by the photocatalytic activity measurement device and gas chromatography (GC). The highest efficiency is observed over the composite GTC (Cr:Gd:Ti = 1:1:1). On the basis of photocurrent measurements and PL, a mechanism for the enhanced photocatalytic activity has been discussed [59]. Unusual magnetic properties of nanocrystalline orthoferrite, GdFeO3, synthesized by conventional solid-state reaction (SSR) route based on the stoichiometric mixing of Fe2O3 and Gd2O3 have been found in the report [60]. The polycrystalline samples of GdFe1-xNixO3(x = 0.0, 0.1) are prepared by solid-state reaction route. It was also noticed that Ni3+ ion substitution results in lattice contraction and enhancement in a dielectric constant, tangent loss, and AC conductivity [61].
The only available magnetic studies were focused on the Mossbauer spectrometry to probe field-induced SR transitions in DFO [62, 63]. Among these compounds, DFO is the only rare earth orthoferrites that show the Morin transition at 35 K followed by three anomalous transitions at temperatures 77 K, 130 K, and 270 K originating probably due to the field-induced spin reorientation (SR) effect originating from the competing magnetic interaction between Dy3+ and Fe3+ ions. Microwave-assisted synthesis of rare earth chromites and physical properties were reported. Magnetization measurements showed that the Neel temperature for antiferromagnetic Cr3+-Cr3+ ordering strongly depends on the RE3+ ionic radius and a rich variety of different magnetic spin interactions exists. On sintered pellets the electronic differences at grain boundary and interior bulk material, which gives the two dielectric relaxations monitered by dielectric spectroscopy. X-ray diffraction, Raman spectroscopy, and temperature-dependent dielectric permittivity data do not indicate potential non-centrosymmetry in the crystal or concomitant ferroelectricity. Systematic efforts have been carried out to prepare full series of (RE)CrO3 compounds, that may resemble in structure of YCrO3 compound. Detailed investigation of the magnetic and dielectric properties and their correlations with a particular focus on possible magnetoelectric or multiferroic behavior as observed was reported [64]. The charge transport properties in (RE)CrO3 materials have been claimed to involve p-type semiconductivity with sensitivity towards humidity, methanol, ethanol, and several gases, which is useful for potential sensor applications. [65, 66]. Furthermore, LaCrO3 and its doped variants are candidates for application as interconnected materials in solid oxide fuel cells [67, 68] and as catalysts for hydrocarbon oxidation [69]. Rare earth orthoferrites of the type LnFeO3 (Ln ¼ Gd, Dy, Sm) are crystallizing the orthorhombically distorted perovskite structure. The presence of electric polarization in the weakly ferromagnetic state of DyFeO3 was reported in a polycrystalline sample, [70] wherein ferroelectricity disappears below the spin reorientation temperature. The importance of the local field induced on Dy ions by the weak ferromagnetic moment of the Fe sublattice in the G4 structure is revealed by the zero-field [71] Fe Mossbauer spectra of DyCrO3. Magnetic susceptibility of heavy rare earth orthochromites at higher temperature [72] and magnetocaloric properties of rare earth-substituted DyCrO3 have also been reported [73]. The detailed investigation of magnetic interaction was found in DyCrO3 bulk powders [74] using hydrothermal synthesis method. Detailed studies on nanocrystalline CeCrO3 were found to exhibit multifunctionalities such as antiferromagnetism, relaxor behavior, and an optical band gap in the visible region. This newly developed synthesis route opens the immense possibilities of preparation of the hitherto unknown Ce3+-based mixed oxides, analogous to other rare earth (RE3+) counterparts [75]. The field-induced metastable state with electric polar order appears at the magnetic ordering temperatures of Cr3+ ions in the weakly ferromagnetic rare earth orthochromites (RCrO3, where R is a magnetic rare earth ion), exhibiting a relatively large electric polarization ~ 0.2–0.8 μC/cm2, starting at rather high temperatures (~ 120–250 K) corresponding to the Neel temperatures of the Cr subsystem [76]. Static and dynamic magnetic properties and effect of surface chemistry on the morphology and crystallinity of DyCrO3 nanoplatelets have been reported [77].
It was also reported that nanosized orthoferrites can be used as photocatalysts in the decomposition of water or the degradation of dyes under light irradiation. This area of research has been enlarged significantly due to the advent of a novel class of oxides exhibiting interesting multiferroic and magnetoelectric properties arising from magnetically induced ferroelectricity. Interestingly, these materials are simple transition metal oxides, most of them possessing the perovskite structure. Novel features of multiferroic and magnetoelectric ferrites and chromites exhibiting magnetically driven ferroelectricity. It has been seen that almost all oxide semiconductor photocatalysts are stable but active under UV light irradiation. Developing a general mild method to prepare rare-earth chromites of uniform crystal size and shape is important for further single crystal related applications. The micrometer-sized single crystals preserve more of the bulk properties compared with their corresponding polycrystalline counterparts acquired with high-temperature treated precursors. Understanding crystal structures and band structures of complex metal oxides is without doubt a key aspect to explore new or improved functionalities. For low-temperature reactions, in particular, the topochemical ones, equally important is the understanding of the factors to direct final structures during a reaction, such as intermediate phase and ion-migration route, by utilizing both kinetic and thermodynamic considerations. In addition, such knowledge, as demonstrated here by the thin film work, will definitely help in developing new ion conductors toward low-temperature applications. The macroporous walls are composed of rare earth orthoferrite nanoparticles, and these hierarchically porous materials show high catalytic activities for the CO+NO reaction, and NO can be fully converted to N2 at temperatures as low as 350°C, indicating their potential in the catalytic conversion of automotive exhaust gas and other catalysis-related fields. This synthesis strategy is a facile method for the preparation of hierarchical porous materials and may give us a guideline for the synthesis of functional materials with further catalytic applications [78]. With the development of the automobile industry, automobile exhaust gas has become one of the major sources of air pollution. The control of automobile exhaust pollution is particularly significant for reducing air pollution. TbFeO3 compounds which possess space group Pbnm may have antiferromagnetic interactions by the presence of Fe spin ions in one direction and the ferromagnetic in other direction with the (TN) Neel temperature of 650 K [79, 80]. The work that has been found for synthesis characterization and the properties of TbFeO3 compound needs to be explored much more as compared to other rare earth oxide ferrites [81,82,83]. The choice to select the atom at A site has become an important concern and may be related with leakage and the loss of multiferroic nature. The structures and magnetic phase transitions in the Mn-doped orthoferrite TbFeO3 studied by neutron powder diffraction have been reported [84].
Ternary Metal Oxide Nano-Material Applications
The application of multiferroic materials is expected from the data values of polarization and magnetization with the existence of magnetoelectric coupling. This could be the main reason that these interesting materials have to be considered in today’s research of solid state physics and chemistry and may utilize in electronic memory and optical transducer devices [85,86,87]. These materials not only possess the memory capacity but may also have sensing properties with magnetic and electronic nature. Multiferroic materials need to be explored further for novel devices by reducing thermal noise for the use of capacitive reading and can replace the magnetoresistive materials [88]. These magnetic-related properties are more sensitive than conventional resistive measurements that allow the magnetic bit density and posses four state memory property [89] which was demonstrated by the encoded information with the help of polarization and magnetization that too measured by resistance measurements. Many nanostructured and nanoscale coating materials have been suggested as possible friction modifying agents, such as carbides, nitrides, metals, and various ceramics. In conclusion, nanotechnology helps to create vehicles possessing properties to endure the harsh conditions of space. Both magnetic and electric properties have the advantage to store data that could be written electrically and read magnetically. This advantages of multiferroic avoid the generation of large load fields to write and read problems [90]. Fe-RAMS devices have been designated using the concept of ferroelectric writing and ferromagnetic reading, and the retained non-volatile memory has been increased thousand times and even more by the use of the same materials at nano-regime. Thus, nanomaterials having such multiferroic properties have tremendous applications in all devices such as memory, sensory, and optical. The size-dependent unconventional multiferroic compounds in nanodots having emerging magnetic properties along with ferroelectric properties were reported. The nanometric size with nonstoichiometric induces the ferromagnetism with host ferroelectric phase and is susceptible to surface morphology that enables to control the properties at the nanoscale [91]. The magnetoelectric coefficients increase on reducing the particle size and could be related with high strain and suppression of spin spiral structure. The electric and magnetic properties of Bi0.90Tb0.10FeO3 nanoparticles depend on the particle sizes and were revealed high as the particle size decreases [92]. In case of Bi2Fe4O9 polycrystalline, the magnetic and ferroelectric properties were investigated with different grain size [93]. Grain size effects the decrease of the ferromagnetic part, but the antiferromagnetic component part dominates as the size increases and shifts the Neel temperature to a higher value. Ferroelectric properties lead to non-volatile data storage devices and high demand in ultrafast electronic instruments which are portable and have high density to storage with less power consumption. Therefore, it is essential to fabricate and to develop such multiferroic nanomaterials which have high sensitivity and efficiency and have a bulk of applications in all segments of machines.
Conclusion
Multiferroic ABO3 type compounds have been focused in the present review based on their structure, composition, and contribution to ferroelectric and ferromagnetic properties. The various factors that improve or decrease the multiferroic properties were taken into consideration. The significant efforts for the synthesis and development of ABO3-based perovskite multiferroic compounds were also mentioned. We attempted to give the outline of specific ternary metal oxide multiferroic compounds that may include bismuth ferrites, yttrium magnates, and rare earth oxides. These ABO3 multiferroic compounds have a lot of applications such as in microelectronic devices, sensors, and storage devices. It is not impossible but rather it is hard to get the breakthroughs of multiferroic compounds in the field of commercialization, and this kind of expectation is expected with the help of research that these productive insights will come soon. It could take further time to develop new materials to achieve the applications in other areas such as magnetoelectric sensors and magnetometers or antennas. There is always a room for improvement of these multiferroic materials and has a lot of market potential in magnetic anomaly detection, navigation, and biomagnetic sensing. If these multiferroic materials are successfully prepared, developed and then commercialized, it will be a breakthrough or huge impact on everyday life and people may choose to stay in academia, join industry, or even start up new businesses.
Abbreviations
- AC:
-
Alternating current
- DFO:
-
Dysprosium ferrite oxides
- DM:
-
Dzyaloshinskii-Moriya
- GC:
-
Gas chromatography
- Hc:
-
Coercive field
- Mr :
-
Remanent magnetization
- MRI:
-
Magnetic resonance imaging
- Ms :
-
Saturation magnetization
- Pr:
-
Remanent polarization
- Ps:
-
Saturation polarization
- RE:
-
Rare earth
- SR:
-
Spin reorientation
- SSR:
-
Solid state reaction
- TC:
-
Curie temperature
- TN:
-
Neel temperature
References
Wohlfarth EP, KHJ B (eds) (1986) Handbook of magnetic materials, vol 2. Elsevier
Guimarães AP, Guimaraes AP (2009) Principles of nanomagnetism, vol 7. Springer, Berlin
Zhou XJ (2007) Magnetism in medicine: a handbook, second completely revised and enlarged edition. Medical Phys 34:4978–4978
Akbarzadeh A, Samiei M, Davaran S (2012) Magnetic nanoparticles: preparation, physical properties, and applications in biomedicine. Nanoscale Res Lett 7:144
Gan S, Lin Y, Feng Y, Shui L, Li H, Zhou G (2018) Magnetic polymeric nanoassemblies for magnetic resonance imaging-combined cancer theranostics. Int J Nanomedicine 13:4263
Dagata F, Ruffinatti F, Boschi S, Stura I, Rainero I, Abollino O, Cavalli R, Guiot C (2018) Magnetic nanoparticles in the central nervous system: targeting principles, applications and safety issues. Molecules 23:9
Stohr J, Siegmann HC (2006) Magnetism. Springer, Berlin, pp 1–808
Blundell S (2010) Magnetism in condensed matter. Oxford University Press, pp 1–238
Dennis CL, Borges RP, Buda LD, Ebels U, Gregg JF, Hehn M, Jouguelet E, Ounadjela K, Petej I, Prejbeanu IL, Thornton MJ (2002) The defining length scales of mesomagnetism: a review. J Phys 14:1175
Kanzig W (1957) Ferroelectrics and antiferroeletrics. In solid state physics. Academic Press 4:1–197
Schmidt VH (1987) Review of order-disorder models for Kdp-family crystals. Ferroelectrics. 72:157–173
Cohen RE (1992) Origin of ferroelectricity in perovskite oxides. Nature 358:136–138
Cohen R (2000) Theory of ferroelectrics: a vision for the next decade and beyond. J Phys Chem Solids 61:139–146
Fatuzzo E, Merz WJ (1967) Ferroelectricity. North-Holland Pub. Co
Wei J, Zhao Y, Li H, Li G, Pan J, Xu D, Zhao Q, Yu D (2014) Hysteresis analysis based on the ferroelectric effect in hybrid perovskite solar cells. J Phys Chem Lett 5:3937–3945
Kalinin SV, Morozovska AN (2015) Multiferroics: focusing light on flexoelectricity. Nat Nanotechnol 10:916
Schmid H (1994) Multi-ferroic magnetoelectrics. Ferroelectrics 162:317–338
Schmid H (2012) The Dice-Stone, der Würfelstein: some personal souvenirs around the discovery of the first ferromagnetic ferroelectric. Ferroelectrics 427:1–33
Schmid H (1999) On the possibility of ferromagnetic, antiferromagnetic, ferroelectric and ferroelastic domain reorientations in magnetic and electric fields. Ferroelectrics 221:9–17
Khomskii D (2009) Classifying multiferroics: mechanisms and effects. Physics 2:1–8
Martin LW, Crane SP, Chu YH, Holcomb MB, Gajek M, Huijben M, Yang CH, Balke N, Ramesh R (2008) Multiferroics and magneto-electrics: thin films and nanostructures. J Phys Cond Matt 20:434220
Martin LW, Ramesh R (2012) Multiferroic and magnetoelectric heterostructures. Acta Materialia 60:2449–2470
Eerenstein, W, Wiora, M, Prieto JL, Scott JF, Mathur ND (2007) Giant sharp and persistent converse magnetoelectric effects in multiferroic epita heterostructures. Nature materials 6:348.
Spaldin AN, Fiebig M (2005) The renaissance of magnetoelectric multiferroics. Science 309:391–392
Scott FJ (2007) Data storage: multiferroic memories. Nat mater 6:256257
Van Aken BB, Jean-Pierre R, Schmid H, Fiebig M (2007) Observation of ferrotoroidic domains. Nature 449:702–705
Jiang J, Bai ZL, Chen ZH, He L, Zhang DW, Zhang QH, Sh Et JA (2018) Temporary formation of highly conducting domain walls for non-destructive read-out of ferroelectric domain-wall resistance switching memories. Nat mater 17:49–56
Mundy JA, Brooks CM, Holtz ME, Moyer JA, Das H, Rébola AF, Heron JT, Clarkson JD, Disseler SM, Liu Z, Farhan A (2016) Atomically engineered ferroic layers yield a room-temperature magnetoelectric multiferroic. Nature 537:523–552
Kan CYD, Takeuchi I, Nagarajan V, Seidel J (2012) Doping BiFeO3: approaches and enhanced functionality. Phys Chem Chem Phys 14:15953–15962
Ederer C, Spaldin NA (2005) Weak ferromagnetism and magnetoelectric coupling in bismuth ferrite. Phys Rev B 71:060401
Wadati H, Okamoto J, Garganourakis M, Scagnoli V, Staub U, Yamasaki Y, Nakao H, Murakami Y, Mochizuki M, Nakamura M, Kawasaki M, Tokura Y (2012) Origin of the large polarization in multiferroic YMnO3 thin films revealed by soft and hard X-ray diffraction. Phys Rev Lett 27:171803
Zhao N, Fan H, Ren X, Ma J, Bao J, Guo Y, Zhou Y (2018) Dielectric, conductivity and piezoelectric properties in (0.67-x)BiFeO3-0.33BaTiO3-xSrZrO3 ceramics. Ceram Int 44:18821–18827
Ren X, Fan H, Zhao Y, Liu Z (2016) Flexible lead-free BiFeO3/PDMS-based nanogenerator as piezoelectric energy harvester. ACS Appl Mater Interfaces 8:26190–26197
Zhao Y, Fan H, Liu G, Liu Z, Ren X (2016) Ferroelectric, piezoelectric properties and magnetoelectric coupling behavior in aurivillius Bi5Ti3FeO15 multiferroic nanofibers by electrospinning. J Alloy Comp 675:441–447
Liu K, Fan H, Ren P, Yang C (2011) Structural, electronic and optical properties of BiFeO3 studied by first-principles. J Alloys Comp 509:1901–1905
Van Aken BB, Palstra TTM, Filippetti A, Spaldin NA (2004) The origin of ferroelectricity in magnetoelectric YMnO3. Nat Mater 3:164–170
Shang M, Zhang C, Zhang T, Yuan L, Ge L, Yuan H, Feng S (2013) The multiferroic perovskite YFeO3. Appl Phys Lett 102:3062903
Zimmermann AS, Van Aken BB, Schmid H, Rivera JP, Li J, Vaknin D, Fiebig M (2009) Anisotropy of antiferromagnetic 180° domains in magnetoelectric LiMPO4 (M = Fe, Co, Ni). Eur Phys J B 71:355
Geller S, Wood EA (1956) Crystallographic studies of perovskite-like compounds. I. Rare earth orthoferrites and YFeO3, YCrO3, YAlO3. Acta Crystallogr 9:563–568
Ye Y, Fan H, Li J (2010) Fabrication and texture evolution of hexagonal YMnO3 nanofibers by electrospinning. Mater Lett 64:419–421
Jain P, Ramachandran V, Clark RJ, Zhou HD, Toby BH, Dalal NS, Kroto HW, Cheetham AK (2009) Multiferroic behavior associated with an order-disorder hydrogen bonding transition in metal−organic frameworks (MOFs) with the perovskite ABX3 architecture. J Am Chem Soc 131:13625–13627
Ahmad T, Lone IH, Ansari SG, Ahmed J, Ahamad T, Alshehri SM (2017) Multifunctional properties and applications of yttrium ferrite nanoparticles prepared by citrate precursor route. Mater Design 126:331–338.
Lee JH, Jeong YK, Park JH, Oak M, Jang HM, Son JY, Scott JF (2011) Spin canting-induced improper ferroelectricity and spontaneous magnetization reversal in SmFeO3. Phys Rev Lett 107:5117201
Bas B, Van A, Thomas TM, Palstra FA, Nicola AS (2004) The origin of ferroelectricity in magnetoelectric YMnO3. Nat Mater 3:164–170
Tofield BC, Fender BEF (1970) Covalency parameters for Cr3+, Fe3+ and Mn4+ in an oxide environment. J Phys Chem Solids 31:2741–2749
Serrao CR, Kundu AK, Krupanidhi SB, Waghmare UV, Rao CNR (2005) Biferroic YCrO3. Phys Rev B 72:220101
Ramesha K, Llobet A, Proffen T, Serrao CR, Rao CNR (2007) Observation of local non-centrosymmetry in weakly biferroic YCrO3. J Phys Condens Matter 19:102202
Duran A, Lopez AMA, Martinez EC, Guaderrama MG, Moran E, Cruz MP, Fernandez F, Franco MAA (2010) Magneto-thermal and dielectric properties of biferroic YCrO3 prepared by combustion synthesis. J Solid State Chem 183:1863–1871
Zhang Y, Zheng A, Yang X, He H, Fan Y, Yao C (2012) Cubic GdFeO3 particle by a simple hydrothermal synthesis route and its photoluminescence and magnetic properties. Cryst Eng Comm 14:8432–8439
Tokunaga Y, Iguchi S, Arima T, Tokura Y (2008) Magnetic-field-induced ferroelectric state in DyFeO3. Phys Rev Lett 101:097205
Gedanken A, Bhattacharya D, Brukental I, Yeshurun Y, Zhong W, Du YW, Felner I, Nowik I, Sivakumar M (2004) Sonochemical synthesis of nanocrystalline rare earth orthoferrites using Fe(CO)5 precursor. Chem Mater 16:3623–3632
Wiglusz RJ, Kordek K, Małecka M, Ciupa A, Ptak M, Pazik R, Pohl P, Kaczorowskia D (2015) A new approach in the synthesis of La1-xGdxFeO3 perovskite nanoparticle structural and magnetic characterization. Dalton Trans 44:20067–20074
Kharton V, Yaremchenko A, Kovalevsky A, Viskup A, Naumovich E, Kerko P, Membr J (1999) Perovskite-type oxides for high-temperature oxygen separation membranes. J Memb Sci 163:307–317
Traversa E, Matsushima S, Okada G, Sadaoka Y, Sakai Y, Watanabe K (1995) NO2 sensitive LaFeO3 thin films prepared by rf sputtering. Sens Actuators B 25:661–664
Bedekar V, Jayakumar OD, Manjanna J, Tyagi AK (2008) Synthesis and magnetic studies of nano-crystalline GdFeO3. Mater Lett 62:3793–3795
Remeika JP (1960) GaFeO3: a ferromagnetic-piezoelectric compound. J Appl Phys 31:263–264
Mathur S, Veith M, Rapalaviciute R, Shen H, Goya GF, Filho WLM, Berquo TS (2004) Molecule derived synthesis of nanocrystalline YFeO3 and investigations on its weak ferromagnetic behavior. Chem Mater 16:1906–1913
Mathur S, Shen H, Lecerf N, Kjekshus A, Fjellvaag H, Goya GF (2002) Nanocrystalline orthoferrite GdFeO3 from a novel heterobimetallic precursor. Advan Mater 14:1405–1409
Parida KM, Nashim A, Mahanta SK (2011) Visible-light driven Gd2Ti2O7 / GdCrO3 composite for hydrogen evolution. Dalton Trans 40:12839–12845
Monndal O, Hossain SKM, Roy B, Pal M (2011) Unusual magnetic properties of nanocrystalline GdFeO3 prepared by solid state reaction route at lower temperature. Funct Mater Lett 04:249–253
Kaur P, Sharma KK, Kumar R, Pandit R (2013) Effect of Ni doping on structural and dielectric properties of GdFeO3. Int J Modern Phys 22:179–183
Prelorendjo LA, Johnson CE, Thomas MF, Wanklyn BM (1980) Spin reorientation transitions in DyFeO3 induced by magnetic fields. J Phys C Solid State Phys 13:2567–2578
Johnson CE, Prelorendjo LA, Thomas MF (1980) Field induced spin reorientation in orthoferrites DyFeO3, HoFeO3 and ErFeO3. J Magn Magn Mater 15:557–558
Gonjal JP, Schmidt R, Ávila D, Amador U, Morán E (2012) Structural and physical properties of microwave synthesized orthorhombic perovskite erbium chromite ErCrO3. J Eur Cer Soc 32:611–618
Russo N, Mescia D, Fino D, Saracco G, Specchia V (2007) N2O decomposition over perovskite catalysts. Ind Eng Chem Res 46:4226–4231
Tsushima K, Aoyagi K, Sugano S (1970) Magnetic and magneto-optical properties of some rare-earth and yttrium orthochromites. J Appl Phys 41:1238–1240
Fergus JW (2004) Lanthanum chromite-based materials for solid oxide fuel cell interconnects. Sol Stat Ion 171:1–15
Gonjal JP, Arevalo-Lo JAM, Moran E (2011) Microwave-assisted synthesis: a fast and efficient route to produce LaMO3 (M= Al, Cr, Mn, Fe, Co) perovskite materials. Mater Res Bull 46:222–230
Beckers J, Rothenberg G (2005) Hot spot hydrocarbon oxidation catalysed by doped perovskites towards cleaner diesel power. Chem Phys Chem 6:223–225
Rajeswaran B, Sanyal D, Chakrabarti M, Sundarayya Y, Sundaresan A, Rao CNR (2003) Interplay of 4f-3d magnetism and ferroelectricity in DyFeO3Europhys. Lett 101:17001
Shireen A, Saha R, Mandal P, Sundaresan A, Rao CNR (2011) Multiferroic and magnetodielectric properties of the Al1-xGaxFeO3 family of oxides. J Mater Chem 21:57–59
Lal HB, Gaur K, Dwivedi RD (1995) Magnetic susceptibility of heavy rare-earth orthochromites at higher temperature. J Mater Sci Lett 14:9–11
McDannald A, Jain M (2015) Magnetocaloric properties of rare-earth substituted DyCrO3. J App Phys 118:043904
McDannald A, Kuna L, Seehra M, Jain M (2015) Magnetic exchange interactions of rare-earth-substituted DyCrO3 bulk powders. Phys Rev B 91:224415
Shukla R, Bera AK, Yusuf SM, Deshpande SK, Tyagi AK, Hermes W, Eul M, Pottgen R (2009) Multifunctional nanocrystalline CeCrO3: antiferromagnetic, relaxor, and optical properties. J Phys Chem C 113:12663–12668
Rajeswaran B, Khomskii DI, Zvezdin AK, Rao CNR, Sundaresan A (2012) Field-induced polar order at the Néel temperature of chromium in rare-earth orthochromites: interplay of rare-earth and Cr magnetism. Phys Rev B 86:214409
Gupta P, Bhargava R, Das R, Poddar P (2013) Static and dynamic magnetic properties and effect of surface chemistry on the morphology and crystallinity of DyCrO3 nanoplatelets. RSC Adv 3:26427–26432
Zhang S, Chen L, Zhou S, Zhao D, Wu L (2010) Facile synthesis of hierarchically ordered porous carbon via in situ self-assembly of colloidal polymer and silica spheres and its use as a catalyst support. Chemistry of Materials 22:3433–3440
Rado GT, Suhl H (2013) Spin arrangements and crystal structure, domains, and micromagnetics: a treatise on modern theory and materials. Eds Academic Press
Artyukhin S, Mostovoy M, Jensen NP, Le D, Prokes K, de Paula VG, Bordallo HN, Maljuk A, Landsgesell S, Ryll H, Klemke B, Paeckel S, Kiefer K, Lefmann K, Kuhn LT, Argyriou DN (2012) The geologically recent giant impact basins at Vesta’s south pole. Science 11:694–697
Gordan JD, Gordodetsky G, Hornreich RM (1976) Magnetization studies of TbFeO3. J Magn Magn Mater 3:288–294
Sivakumar M, Gedanken A, Bhattacharya D, Brukental I, Yeshurun Y, Zhong W, Du YW, Felner I, Nowik I (2004) Sonochemical synthesis of nanocrystalline rare earth orthoferrites using Fe(CO)5 precursor. Chem Mater 16:3623–3632
Guan-Jun L, Hua Y, Tao X (2012) Polyacrylamide gel synthesis and visible-light photocatalytic activity of TbFeO3 nanoparticles chem. J Chinese U 33:1565
Nair HS, Chatterji T, Kumar CMN, Hansen T, Nhalil H, Elizabeth S, Strydom AM (2016) Magnetic structures and magnetic phase transitions in the Mn-doped orthoferrite TbFeO3 studied by neutron powder diffraction. Appl Phys 119:053901
Zhu MW, Ye GZ (2004) Effects of chemical modification on the electrical properties of 0.67 BiFeO3-0.33 PbTiO3 ferroelectric ceramics. Ceram Int 30:1435–1442
Seshadri R, Hill AN (2001) Visualizing the role of Bi 6s “lone pairs” in the off-center distortion in ferromagnetic BiMnO3. Chem Mater 13:2892–2899
Subramanian MA, He T, Chen J, Rogado NS, Calvarese TG, Sleight AW (2006) Giant room–temperature magnetodielectric response in the electronic ferroelectric LuFe2O4. Adv Mater 18:1737–1739
Gajek M, Bibes M, Fusil S, Bouzehouane K, Fontcuberta J, Barthelemy A, Fert A (2007) Tunnel junctions with multiferroic barriers. Nat Mater 6:296
Eerenstein W, Mathur ND, Scott JF (2006) Multiferroic and magnetoelectric materials. Nature 442:759
Ma J, Hu J, Li Z, Nan CW (2011) Recent progress in multiferroic magnetoelectric composites: from bulk to thin films. Advan Mater 23:1062–1087
Xu T, Shimada T, Uratani Y, Wang X, Wang J, Kitamura T (2017) Multiferroic phases and transitions in ferroelectric lead titanate nanodots. Sci Rep 7(45373):1–8
Dhir G, Uniyal P, Verma NK (2016) Effect of particle size on the multiferroic properties of Tb-doped BiFeO3 nanoparticles. J Supercond Nov Magn 29:2621–2628
Tian ZM, Yuan SL, Wang XL, Zheng XF, Yin SY, Wang CH, Liu L (2009) Size effect on magnetic and ferroelectric properties in Bi2Fe4O9multiferroic ceramics. J App Phy 106:103912–103914
Acknowledgements
IHL thanks to all other authors for critical reading and fruitful discussions during the preparation of this manuscript.
Funding
The authors declare that they have no financial regard.
Availability of Data and Materials
Not applicable
Author information
Authors and Affiliations
Contributions
IHL designed the structure, drafted the manuscript, and provided the overall supervision of the work. JA and NRER collected the literature and contributed to the scientific discussions. AHB contributed to the further refinement of the manuscript. AFAA contributed to the final version of the manuscript. AA participated in the sequence alignment and contributed to the biological part. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Authors’ Information
Not applicable
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Lone, I.H., Aslam, J., Radwan, N.R.E. et al. Multiferroic ABO3 Transition Metal Oxides: a Rare Interaction of Ferroelectricity and Magnetism. Nanoscale Res Lett 14, 142 (2019). https://doi.org/10.1186/s11671-019-2961-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11671-019-2961-7