Abstract
A ternary and composite MoIn2S4@CNTs counter electrode (CE) with a hedgehog ball structure was synthesized by using a facile one-step hydrothermal method. The composite MoIn2S4@CNTs film possesses large specific surface area through N2 adsorption-desorption isotherms test, which is advantageous to adsorb more electrolyte and provide larger active contact area for the electrode. In addition, the composite MoIn2S4@CNTs CE exhibits low charge transfer resistance and fine electrocatalytic ability made from a series of electrochemical tests including cyclic voltammetry, electrochemical impedance, and Tafel curves. Under optimal conditions, the DSSC based on the MoIn2S4@CNTs-2 composite CE achieves an impressive power conversion efficiency as high as 8.38%, which remarkably exceeds that of the DSSCs with the MoIn2S4 CE (7.44%) and the Pt electrode (8.01%). The current work provides a simplified preparation process for the DSSCs.
Similar content being viewed by others
Background
In recent decades, it is urgent to exploit and utilize renewable energy substituting the conventional fossil fuels with the severe energy shortage and environmental degradation increasing [1, 2]. Dye-sensitized solar cell (DSSC) has attracted wide-spread research by virtue of its environmental friendliness, facile preparation process, brilliant photovoltaic performance, and so on [3, 4]. The counter electrode (CE), as one of the significant components of a DSSC, plays the role of gathering electrons from external circuit and catalyzing the reduction reaction of I3− to I− in the liquid electrolyte [5, 6]. Generally, ideal CE materials contain the merits of high electrical conductivity and remarkable catalytic activity. However, platinum (Pt) as a prevalent and efficient CE material is confined to large-scale commercialized application because of the major weaknesses of scarcity, expensiveness, and poorly long-term stability [7, 8]. Therefore, a great many of efforts have been made to develop easily accessible, cost-effective, and Pt-like catalytic activity applied in DSSC for years [9].
Up to now, various kinds of outstanding alternative materials have been proposed, such as carbonaceous materials [10, 11], transition metal chalcogenides [12], conductive polymers [13], metal alloys [14], and their compounds [15, 16]. Among them, the binary transition metal chalcogenides attracted a great deal of attention due to their unique structure and chemical properties. For instance, the synthesized MoS2 on FTO substrate exhibits a sandwich-layered structure, larger surface area, and more active edge sites leading to extremely photoelectric performance as a CE for DSSC [17]. Meanwhile, extensive research work focused on the catalytic activity for I3− reduction also has been made for WS2 [18], FeS2 [19], CoS [20], and NiS2 [21], which were comparable to or even better than that of the Pt electrode. Nevertheless, the inherent characteristic of these materials, such as low electrical conductivity and only two-fixed chemical compositions, hampered further improvement in their catalytic activity [22]. Hence, numerous methods aimed to overcome the abovementioned shortcoming were adopted to synthesize multinary transition metal chalcogenides by component elements adjusting, structure designing, and morphology tailoring. Fortunately, considerable multinary transition metal chalcogenides have achieved significant enhancements in catalytic ability for DSSCs, such as NiCo2S4 [23], MIn2S4 (M = Fe, Co, Ni) [22], CuInS2 [24], CoCuWSx [25], and Ag8GeS6 [26], which catalytic ability are obviously much better than that of their binary counterparts.
Moreover, it is widely acknowledged that carbon nanotubes (CNTs) exhibit considerably novel characteristics of large specific surface area, superb electrical conductivity, high mechanical strength, and photochemical stability, which are widely used in the synthesis and modification of other materials [27]. Regrettably, CNTs show poor electrocatalytic activity for I3− reduction, which greatly limits their application independently in a DSSC device. Fortunately, a large number of studies have shown that the composite CE modified with CNTs all obtained greatly improved photoelectric performance for DSSCs [18, 28, 29]. Liu et al. have reported a flower-like hierarchical structure of Cu2MnSnS4/CNT (CMTS/CNT) CE via solvothermal method in DSSC gained a photoelectric conversion efficiency of 8.97%, much higher than that of the DSSCs with CMTS (6.21%) and Pt (8.37%) CEs [29].
Based on the above considerations, in this study, a MoIn2S4@CNT composite CE of DSSC with hedgehog ball structure was synthesized by using a facile one-step hydrothermal method and expected to improve higher device performance. Scanning electron microscope results show that different CNT contents result in visible changes on morphology. According to a series of electrochemical characterizations including cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS), and Tafel curves tests, the MoIn2S4@CNTs CE indicates a remarkable catalytic activity and fine charge transfer resistance. The DSSC assembled with the MoIn2S4@CNTs CE with suitable content achieves a superior power conversion efficiency of 8.38%, which is better than that of the DSSC based on the Pt CE (8.01%).
Methods
Materials
Sodium molybdate dihydrate (Na2MoO4·2H2O), indium chloride tetrahydrate (InCl3·4H2O), and thioacetamide (TAA) were purchased from Shanghai Chemical Agent Ltd., China, which were used directly without further purification. Carbon nanotubes (CNTs) were gained from Aladdin Chemical Agent Ltd., China. The commercial Z907 dye was obtained from Solaronix Ltd. (Switzerland). The fluorine-doped SnO2 (FTO) glass, purchased from NSG, Japan (15 Ω sq−1), were cleaned with detergent and acetone as well as ethyl alcohol in sequence after cutting into squares of 1.5 cm × 2.0 cm.
Preparation of porous TiO2 photoanodes
The colloid of TiO2 was prepared as our previous work [30]. The dye-sensitized TiO2 photoanodes were fabricated as follows: Firstly, 3-M tape (50 μm thick) with exposed area of 0.283 cm2 was attached on FTO. Subsequently, the as-prepared TiO2 colloid was coated on by using a blade-coating method. Secondly, the drying TiO2 electrode was sintered at 450 °C for 30 min in muffle furnace. Afterwards, the TiO2 electrode was immersed in a 40-mM titanium tetrachloride (TiCl4) aqueous solution at 70 °C for 30 min, and then annealed in air at 450 °C for 30 min. After cooling down to room temperature, the TiO2 electrode was immersed in dye Z907 (0.3 mM) absolute ethanol solution for 24 h to adsorb sufficient dyes and obtained the resultant dye-sensitized TiO2 photoanode.
Fabrication of ternary MoIn2S4@CNTs CE
The MoIn2S4 thin films were grown directly onto FTO substrates by a simple approach referring to our previous report [31]. In a typical preparation, 0.0696 g Na2MoO4·2H2O, 0.169 g InCl3·4H2O, and 0.1394 g TAA were diffused in 30 ml deionized water under ultrasonicating for 2 h until all the reactants were dissolved. The pre-cleaned FTO substrates were put into a 100-ml Teflon-lined stainless steel autoclave with the conductive side facing up before transferring the above precursor mixture into it. After being sealed, the autoclave was placed in an oven and heated under 200 °C for a reaction time of 15 h. The FTO glass substrates covered with MoIn2S4 materials were taken out from the autoclave, washed with ethanol, deionized water, and then dried in air under 60 °C for 12 h.
In order to study the impact of the CNT contents on the fabricated composite CE and the performance of the DSSC, different contents of CNTs adding to the precursor were conducted, including three samples in which the amount of CNTs were 10, 20, and 30 mg, respectively, keeping the other reagents and fabrication processes unchanged. The above samples were marked MoIn2S4 (0 mg), MoIn2S4@CNTs-1 (10 mg), MoIn2S4@CNTs-2 (20 mg), and MoIn2S4@CNTs-3 (30 mg).
For comparison, a pyrolysised Pt CE was employed as the reference CE. The H2PtCl6 in isopropanol solution (0.50 wt%) was dropped onto the surface of the FTO glass, and then sintered at 450 °C in muffle furnace for 30 min to fabricate the Pt CE.
Fabrication of the DSSCs
The DSSCs with sandwich structure were constructed by clipping the sample CEs (including MoIn2S4, various MoIn2S4@CNTs, and Pt CEs) together with as-prepared dye-sensitized TiO2 photoanode. Surlyn was used as spacer between the electrodes and followed by filling the interspace with liquid redox electrolyte that consisted of 0.60 M tetrabutylammonium iodide, 0.10 M lithium iodide, 0.05 M iodine, and 0.50 M 4-tert-butyl-pyridine acetonitrile solution.
Characterizations
Chemical element composition of the samples was characterized by using X-ray photoelectron spectroscopy (XPS) analysis (Kratos Axis Ultra). The morphological features of samples were observed by field emission scanning electron microscopy (FESEM, JSM-7001F). BET-specific surface area method was employed using a JW-K analyser by nitrogen absorption to test the surface area and pore size distribution. The other relevant electrochemical properties were researched by a CHI660E electrochemical workstation. The electrochemical impedance spectroscopy (EIS) was performed in a frequency range of 0.1–105 Hz with a disturbed amplitude of 5 mV. The photovoltaic performances of the DSSCs were carried out by measuring current density-voltage (J-V) characteristic curves under irradiation of 100 mW cm−2 from the solar simulator (CEL-S500, Beijing China Education Au-light Co., Ltd).
Results and Discussions
Composition and Morphology
The XPS is employed to examine the surface compositions and chemical states of each element in MoIn2S4 and MoIn2S4@CNTs films. The spectrum of survey data in MoIn2S4 and MoIn2S4@CNTs-2 samples is demonstrated in Fig. 1a to verify the presence of Mo, In, S, and C (as a reference) elements. In addition, all spectra of the four samples, calibrated by the C 1s peak at the binding energy of 284.6 eV [17], are analyzed via the Gaussian fitting method showing in Fig. 1b and c. The C 1s peak appeared in MoIn2S4 sample is well known to have originated from the adventitious carbon caused by exposure to the air. In the Mo 3d region, two major peaks at 228.8 and 232.1 eV are assigned to Mo 3d5/2 and Mo 3d3/2 of the MoS2 [32], respectively. This result confirms that Mo element is in its IV oxidation state, which is reduced to Mo4+ (MoIn2S4) from Mo6+ (Na2MoO4) [33]. The doublet peaks at binding energies of 445.2 and 452.2 eV correspond to In 3d5/2 and In 3d3/2 [24, 34]. As for the XPS spectra of S 2p, the peaks located at 161.8 and 163.1 eV belong to S 2p3/2 and 2p1/2, respectively, which is ascribed to the S2− [17, 32]. The above results are well in agreement with our previous studies [31]. Moreover, no other element or extra peak is found in the survey, which reconfirms that the samples synthesized have the similar chemical compositions and structure.
The surface morphologies of as-prepared MoIn2S4 and MoIn2S4@CNTs nanofilms are observed by SEM images in Fig. 2. In Fig. 2a, MoIn2S4 sample displays a petal-like nanosheet structure with a uniform, smooth, and dense surface. Unlike MoIn2S4 nanofilms, the hedgehog ball structure is found in MoIn2S4@CNT samples in Fig. 2b–d, and the average diameter of the MoIn2S4@CNTs nanospheres is around 890 nm. It is easy to observe that so many network nanosheets are fully grown on the FTO substrate. Compared to the MoIn2S4@CNT samples with low (MoIn2S4@CNTs-1) and high (MoIn2S4@CNTs-3) CNT contents, the sample with moderate content (MoIn2S4@CNTs-2) exhibits more hedgehog balls and nanosheets array on the network of the MoIn2S4. A good contact performance between the network nanosheet array structure with large number hedgehog balls on FTO substrate facilitates the reduction of I3− attributed to its good conductivity and vast catalytic active sites, which can be predicted that the MoIn2S4@CNTs-2 CE will achieve better performance than that of the MoIn2S4, MoIn2S4@CNTs-1, and MoIn2S4@CNTs-3 CEs. Moreover, dissimilar morphologies between MoIn2S4 and MoIn2S4@CNTs indicate that CNTs play a pivotal role in controlling morphology of the samples.
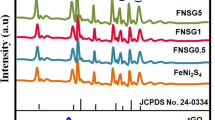
Figure 3 shows the XRD patterns of various samples. Among them, the diffraction peaks at 25.74 and 42.85° attribute to the signals of the CNTs [31]. The diffraction peaks at 2θ = 27.5, 33.4, 43.7, 47.9, 56.2, and 59.6° belong to the (311), (400), (511), (440), (533), and (444) crystallographic plane (JCPDS card no. 32-0456) of In2S3. The peaks at 14.4 and 66.5° are regarded to (002) and (114) crystal planes of cubic structure (JCPDS card no. 37-1492) for the MoS2 [33]. From the MoIn2S4@CNTs-2 and MoIn2S4 samples, the peaks of the above discussed appear well in the both samples. Compared to the MoIn2S4 sample, the peak for the CNTs at 25.74° can be seen clearly in MoIn2S4@CNTs-2 XRD patterns. Thus, it can be inferred that the MoIn2S4@CNTs-2 materials are synthesized successfully and there are no impurities introduction.
N2 adsorption-desorption isotherms are measured and showed in Fig. 4 to explore the specific surface areas and pore features. Generally, the larger specific surface area facilitates more convenient charge transmission on the CE/electrolyte interface [35]. It demonstrates from Fig. 4 that the samples possess an evident hysteresis loop of type IV adsorption-desorption behavior, and their corresponding data calculated from the Brunauer-Emmett-Teller (BET) and Barrett-Joyner-Halenda (BJH) method are tabulated in Table 1. By comparison, it is easy to find that the specific surface area and average pore diameter of the MoIn2S4@CNT samples are much better than that of the MoIn2S4. Among the three MoIn2S4@CNT samples, MoIn2S4@CNTs-2 shows the largest specific surface area of 66.80 m2 g−1 and the smallest average pore diameter of 17.8 nm, which can be ascribed to the excellent hedgehog ball structure after doping of moderate CNTs. It is reasonable to believe that the MoIn2S4@CNTs-2 CE will obtain the fine catalytic activity and hence achieve highly efficient device performance.
Electrochemical properties
CV measurements are carried out to study the electrocatalytic behavior of the as-obtained samples in the potential range of − 0.6 to 1.0 V at a scan rate of 60 mV s−1 for the MoIn2S4 and MoIn2S4@CNTs CEs, and the calculated values are summed up in Table 1. The left peaks of the two pairs of oxidation and reduction peaks in each CV curve in Fig. 5a are ascribed to the equation (I3− + 2e− ↔ 3I−), which determines the performance of electrocatalytic activity of the CE materials, especially in DSSCs [23, 36]. The values of the negative reduction peak current density (Jpc), a key parameter in CV test, follows the orders of Pt (3.80 mA cm−2) < MoIn2S4 (4.31 mA cm−2) < MoIn2S4@CNTs-1 (4.68 mA cm−2) < MoIn2S4@CNTs-3 (5.09 mA cm−2) < MoIn2S4@CNTs-2 (7.47 mA cm−2). Evidently, the MoIn2S4 CE itself has good catalytic activity, and the MoIn2S4@CNTs-2 CE exhibits much higher Jpc than that of the Pt, MoIn2S4 CEs, and the other two kinds of MoIn2S4@CNTs CEs attributed to its distinctive surface morphology, the synergistic effect of CNTs doped, and the larger surface area. The results indicate that the MoIn2S4@CNTs-2 CE has fine electrocatalytic activity for the I−/I3− redox couple in DSSC CEs. Figure 5b shows the CV curves of the MoIn2S4@CNTs-2 CE at the scan rate of 60 mV s−1 and they are almost no changing with 50 cycles, which indicates that the MoIn2S4@CNTs-2 CE possesses excellent electrochemical stability.
Figure 6a–c show the CVs of MoIn2S4@CNTs CEs at different scan rates. With the scan rates increasing from 20 to 120 mV s−1, the oxidation and reduction peaks shift towards positive and negative direction owing to the fast diffusions of I−/I3− redox couple on the surfaces of CEs and the large electrochemical polarization [28]. Furthermore, Fig. 6d shows the relationship between the anodic and cathodic peaks current densities of the left peak pairs against the square root of sweep rates. The well-fitted linear relations indicate that the redox reaction of I−/I3− is dominated by diffusion-controlled ion transport [22, 25].
Figure 7a shows the Nyquist plots of the different samples to further insight into the kinetics of the interfacial charge transfer process. The EIS data made from the fitted curves with a model equivalent circuit of the insert is listed in Table 2. Typically, the Nyquist plots contain two semicircles, the first semicircle on the left represents the charge transfer resistance Rct at the CE and electrolyte interface, and the second semicircle corresponds to the Nernst diffusion impedance in the electrolyte, while the intercept of the curve in the high-frequency region on the real axis is known as the series resistance Rs. In general, Rs and Rct are two vital parameters for evaluating the catalytic activity of CE in DSSC. A small Rs indicates good contact between the catalyst and the substrate, and hence the resistance of the entire device is also small [29, 37, 38]. Meanwhile, little Rct stands for a high charge transfer rate. As presented in Table 2, the Rs values of the MoIn2S4, MoIn2S4@CNTs-1, MoIn2S4@CNTs-2, and MoIn2S4@CNTs-3 CEs are 24.77, 23.16, 18.96, and 19.58 Ω cm2, respectively. Obviously, all the composite MoIn2S4@CNTs CEs have the smaller Rs than that of the MoIn2S4 CE, indicating that the conductivity of the MoIn2S4@CNTs CEs is enhanced after doping CNTs. Furthermore, among the four CEs, the trends of Rct is MoIn2S4 > MoIn2S4@CNTs-1 > MoIn2S4@CNTs-3 > MoIn2S4@CNTs-2, which suggests an inverse order of electrochemical impedance and catalytic ability of the CEs. MoIn2S4@CNTs-2 CE possesses the lowest Rct value can be attributed to the synergy of the CNTs with fine conductivity and MoIn2S4 with excellent catalytic ability, resulting in more effective reduction of triiodide on the CE/electrolyte interface. Apparently, the conductivity and catalytic ability of the MoIn2S4@CNTs composite CEs has been improved greatly than that of the MoIn2S4 CE, and the result is completely consistent with the BET and CV tests.
The Tafel polarization curves of the various CEs are measured as shown in Fig. 7b, and the corresponding parameters values are summarized in Table 2. Normally, a standard Tafel curve includes two significant parameters named exchange current density (J0) and limiting diffusion current density (Jlim). J0 is related to the catalytic reduction reaction. The larger the J0 is, the better the catalytic effect. Jlim is also positively related to the diffusion efficiency of electrolyte. The larger Jlim indicates the faster diffusion of I3− ions [29, 37]. As presented in Fig. 7b and Table 2, the Jlim and J0 are all in orders of Pt < MoIn2S4 < MoIn2S4@CNTs-1 < MoIn2S4@CNTs-3 < MoIn2S4@CNTs-2, suggesting that the catalytic activity of the MoIn2S4@CNTs-2 CE has enhanced greatly after doping CNTs. Among above CEs, the MoIn2S4@CNTs-2 CE gains the best catalytic activity compared to the others. The largest Jlim and J0 of the MoIn2S4@CNTs-2 CE can be ascribed to its large specific surface area made from hedgehog ball structure and enhanced conductivity by CNTs doping.
Photovoltaic performance of the DSSCs
For contrast, the DSSCs with the MoIn2S4, MoIn2S4@CNTs, and Pt CEs are prepared with the uniform photoanodes and electrolyte. The J-V characteristic curves are measured under 1 sun (AM 1.5 G, 100 mW cm−2) and the corresponding photovoltaic parameters values are listed in Table 3. Four key parameters including short-circuit current density (Jsc), open-circuit voltage (Voc), fill factor (FF), and power conversion efficiency (η) are usually adopted to assess the photovoltaic performance of the DSSCs. The FF and η of the DSSCs are calculated according to the Eqs. (1) and (2):
where Pin is the incident light power and Jmax (mA cm–2) and Vmax (V) are the current density and voltage at the point of maximum power output in the J–V curves, respectively.
As can be seen from Fig. 8, the DSSCs with MoIn2S4 and CNTs CEs have power conversion efficiency of 7.44% and 3.62%. Compared to the DSSCs with the MoIn2S4 and CNTs CEs, the DSSCs assembled with the three MoIn2S4@CNTs CEs exhibit improvement Jsc and η values. Moreover, the Jsc and η values of the DSSCs based on the MoIn2S4@CNTs CEs increase with the CNT contents increasing from 10 to 20 mg. While further increases the CNT content to 30 mg, it results in a slight descent for the Jsc and η. Compared with the Pt-based DSSC, all the three MoIn2S4@CNTs-based DSSCs exhibit improved η of 8.16%, 8.31%, and 8.38%, which are higher than that of the Pt-based DSSC (η of 8.01%) under the same condition. Especially, the DSSC assembled with the MoIn2S4@CNTs-2 CE shows the best photovoltaic performance and achieves a η of 8.38%, and its corresponding Jsc of 17.17 mA cm−2, Voc of 0.745 V, and FF of 0.655. The improved photoelectric property of the DSSC with the MoIn2S4@CNTs-2 CE was due to the synergetic effect of the CNTs and the MoIn2S4.
Conclusions
Ternary MoIn2S4 and MoIn2S4@CNTs counter electrodes are fabricated on FTO substrate by using a facile one-step hydrothermal method and served in DSSCs. Under optimal conditions, the DSSCs based on the MoIn2S4@CNTs CEs all achieve good power conversion efficiency. Especially, the DSSC with the MoIn2S4@CNTs-2 composite CE exhibits a good power conversion efficiency of 8.38%, which is much higher than that of the DSSCs with the MoIn2S4 CE (7.44%) and the Pt electrode (8.01%). The enhanced photoelectric property of the DSSC with the MoIn2S4@CNTs-2 CE was due to the synergetic effect of the CNTs and the MoIn2S4. Meanwhile, the synergetic effect of the MoIn2S4@CNTs CE in electrochemical performance has confirmed from a series of electrochemical test including cyclic voltammetry, electrochemical impedance, and Tafel curves. The composite MoIn2S4@CNTs film possesses large specific surface area through N2 adsorption-desorption isotherms test, which is advantageous to adsorb more electrolyte and provide larger active contact area for the electrode. The facts of the MoIn2S4@CNTs CE served in DSSC broadens the potential applications of transition metal complex semiconductors in the field of optoelectronic chemistry.
Availability of Data and Materials
All data sets on which the conclusions of the manuscript rely are presented in the main paper.
Abbreviations
- CE:
-
Counter electrode
- CV:
-
Cyclic voltammetry
- DSSC:
-
Dye-sensitized solar cell
- I−/I3− :
-
Iodide/triiodide
- J 0 :
-
Exchange current density
- J lim :
-
Limiting current density
- J max :
-
Maximum current density
- J sc :
-
Short-circuit current density
- J-V :
-
Photocurrent-photovoltage
- P in :
-
Incident light power
- R ct :
-
Charge transfer resistance
- R s :
-
Series resistance
- SEM:
-
Scanning electron microscopy
- V max :
-
Maximum voltage
- V oc :
-
Open-circuit voltage
References
Dresselhaus MS, Thomas IL (2001) Alternative energy technologies. Nature 414:332–337
Balzani V, Credi A, Venturi M (2008) Photochemical conversion of solar energy. ChemSusChem 1:26–58
O’Regan B, Grätzel M (1991) A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 353:737–740
Hagfeldt A, Boschloo G, Sun L, Kloo L, Pettersson H (2010) Dye-sensitized solar cells. Chem Rev 110:6595–6663
Wu JH, Lan Z, Lin JM, Huang ML, Huang YF, Fan LQ, Luo GG, Lin Y, Xie YM, Wei YL (2017) Counter electrodes in dye-sensitized solar cells. Chem Soc Rev 46:5975–6023
Thomas S, Deepak TG, Anjusree GS, Arun TA, Nair SV, Nair AS (2014) A review on counter electrode materials in dye-sensitized solar cells. J Mater Chem A 2:4474–4490
Theerthagiri J, Senthil AR, Madhavan J, Maiyalagan T (2015) Recent progress in non-platinum counter electrode materials for dye-sensitized solar cells. ChemElectroChem 2:928–945
Briscoe J, Dunn S (2016) The future of using earth-abundant elements in counter electrodes for dye-sensitized solar cells. Adv Mater 28:3802–3813
Yun SN, Hagfeldt A, Ma TL (2014) Pt-free counter electrode for dye-sensitized solar cells with high efficiency. Adv Mater 26:6210–6237
Zhou ZP, Sigdel S, Gong JW, Vaagensmith B, Elbohy H, Yang HJ, Krishnan S, Wu XF, Qiao QQ (2016) Graphene-beaded carbon nanofibers with incorporated Ni nanoparticles as efficient counter electrode for dye-sensitized solar cells. Nano Energy 22:558–563
Yun SN, Hou YZ, Wang C, Zhang YL, Zhou X (2019) Mo2C-based binary and ternary nanocomposites as high-efficiency counter electrodes for dye-sensitized solar cells. Ceram Int 45:15589–15595
Singh E, Kim KS, Yeom GY, Nalwa HS (2017) Two-dimensional transition metal dichalcogenide-based counter electrodes for dye-sensitized solar cells. RSC Adv 7:28234–28290
Xiao YM, Lin JY, Tai SY, Chou SW, Yue GT, Wu JH (2012) Pulse electropolymerization of high performance PEDOT/MWCNT counter electrodes for Pt-free dye-sensitized solar cells. J Mater Chem 22:19919–19925
Chang PJ, Cheng KY, Chou SW, Shyue JJ, Yang YY, Hung CY, Lin CY, Chen HL, Chou HL, Chou PT (2016) Tri-iodide reduction activity of shape- and composition-controlled PtFe nanostructures as counter electrodes in dye-sensitized solar cells. Chem Mater 28:2119–2129
Lou Y, Zhao WJ, Li CG, Huang H, Bai TY, Chen CL, Liang C, Shi Z, Zhang D, Chen XB, Feng SH (2017) Application of Cu3InSnSe5 heteronanostructures as counter electrodes for dye-sensitized solar cells. ACS Appl Mater Interfaces 9:18046–18053
Sigdel S, Dubey A, Elbohy H, Aboagye A, Galipeau D, Zhang L, Fong H, Qiao QQ (2014) Dye-sensitized solar cells based on spray-coated carbon nanofiber/TiO2 nanoparticle composite counter electrodes. J Mater Chem A 2:11448–11453
Liang J, Li J, Zhu HF, Han YX, Wang YR, Wang CX, Jin Z, Zhang GM, Liu J (2016) One-step fabrication of large-area ultrathin MoS2 nanofilms with high catalytic activity for photovoltaic devices. Nanoscale 8:16017–16025
Wu JH, Yue GT, Xiao YM, Huang ML, Lin JM, Fan LQ, Lan Z, Lin JY (2012) Glucose aided preparation of tungsten sulfide/multi-wall carbon nanotube hybrid and sse as counter electrode in dye-sensitized solar cells. ACS Appl Mater Interfaces 4:6530–6536
Wang YC, Wang DY, Jiang YT, Chen HA, Chen CW (2013) FeS2 nanocrystal ink as a catalytic electrode for dye-sensitized solar cells. Angew Chem Int Ed 52:6694–6698
Kung CW, Chen HW, Lin CY, Huang KC, Vittal R, Ho KC (2012) CoS acicular sanorod arrays for the counter electrode of an efficient dye-densitized solar cell. ACS Nano 6:7016–7025
Wan ZQ, Jia CY, Yan W (2015) In situ growth of hierarchical NiS2 hollow microspheres as efficient counter electrode for dye-sensitized solar cell. Nanoscale 7:12737–12742
Hou WJ, Xiao YM, Han GY (2017) An interconnected ternary MIn2S4 (M=Fe, Co, Ni) thiospinel nanosheet array: a type of efficient platinum-ffree counter electrode for dye-sensitized solar cells. Angew Chem Int Ed 56:9146–9150
Khoo SY, Miao JW, Yang HB, He ZM, Leong KC, Liu B, Tan TTY (2015) One-step hydrothermal tailoring of NiCo2S4 nanostructures on conducting oxide substrates as an efficient counter electrode in dye-sensitized solar cells. Adv Mater Interfaces 2:1500384
Chen BK, Chang S, Li DY, Chen LL, Wang YT, Chen T, Zou BS, Zhong HZ, Rogach AL (2015) Template synthesis of CuInS2 nanocrystals from In2S3 nanoplates and their application as counter electrodes in dye-sensitized solar cells. Chem Mater 27:5949–5956
Qian X, Liu HY, Yang JH, Wang HW, Huang J, Xu C (2019) Co–Cu–WSx ball-in-ball nanospheres as high-performance Pt-free bifunctional catalysts in efficient dye-sensitized solar cells and alkaline hydrogen evolution. J Mater Chem A 7:6337–6347
He QQ, Qian TY, Zai JT, Qiao QQ, Huang SS, Li YR, Wang M (2015) Efficient Ag8GeS6 counter electrode prepared from nanocrystal ink for dye-sensitized solar cells. J Mater Chem A 3:20359–20365
Yan Q, Chang ML, Bao SJ, Bao QL (2007) Carbon nanotube/polyaniline composite as anode material for microbial fuel cells. J Power Sources 170:79–84
Yue GT, Wu W, Liu XQ, Zheng HW (2018) Enhanced photovoltaic performance of dye-sensitized solar cells based on a promising hybrid counter electrode of CoSe2/MWCNTs. Sol Energy 167:137–146
Liu XQ, Gao LF, Yue GT, Zheng HW, Zhang WF (2017) Efficient dye-sensitized solar cells incorporating hybrid counter electrode of CuMnSnS4 microsperes/carbon nanotubes. Sol Energy 158:952–959
Yue GT, Wu JH, Xiao YM, Lin JM, Huang ML, Lan Z, Fan LQ (2013) Functionalized graphene/poly(3,4-ethylenedioxythiophene):polystyrenesulfonate as counter electrode catalyst for dye-sensitized solar cells. Energy 54:315–321
Cheng RZ, Gao XM, Yue GT, Fan LQ, Gao YY, Tan FR (2020) Synthesis of a novel MoIn2S4 alloy film as efficient electrocatalyst for dye-sensitized solar cell. Sol Energy 201:116–121
Qian X, Xu C, Jiang YQ, Zhang J, Guan GX, Huang YX (2019) Ni-Co-MoSx ball-in-ball hollow nanospheres as Pt-free bifunctional catalysts for high-performance solar cells and hydrogen evolution reactions. Chem Eng J 368:202–211
Senthilkumar R, Ramakrishnan S, Balu M, Ramamurthy PC, Kumaresan D, Kothurkar NK (2018) One-step hydrothermal synthesis of marigold flower-like nanostructured MoS2 as a counter electrode for dye-sensitized solar cells. J Solid State Electrochem 22:3331–3341
Hou WJ, Xiao YM, Han GY (2018) The dye-sensitized solar cells based on the interconnected ternary cobalt diindium sulfide nanosheet array counter electrode. Mater Res Bull 107:204–212
Yue GT, Wu JH, Xiao YM, Huang ML, Lin JM, Lin JY (2013) High performance platinum-free counter electrode of molybdenum sulfide–carbon used in dye-sensitized solar cells. J Mater Chem A 1:1495–1501
Wu MX, Lin X, Wang YD, Wang L, Guo W, Qi DD, Peng XJ, Hagfeldt A, Grätzel M, Ma TL (2012) Economical Pt-free catalysts for counter electrodes of dye-sensitized solar cells. J Am Chem Soc 134:3419–3428
Liu XQ, Yue GT, Zheng HW (2017) A promising vanadium sulfide counter electrode for efficient dye-sensitized solar cells. RSC Adv 7:12474–12478
Ju MJ, Jeon I, Kim HM, Choi JI, Jung S, Seo J, Choi IT, Kang SH, Kim HS, Noh MJ (2016) Edge-selenated graphene nanoplatelets as durable metal-free catalysts for iodine reduction reaction in dye-sensitized solar cells. Sci Adv 56:e1501459
Acknowledgements
The authors are very grateful to the joint support by NSFC (No. 61704047). This work is also supported by open topic of Fujian Key Laboratory of Photoelectric Functional Materials (FJPFM-201501).
Author information
Authors and Affiliations
Contributions
RC carried out the experiments, participated in the sequence alignment, and drafted the manuscript. XG participated in the device preparation. LF, MY, and YG were involved in the SEM, EIS, and CV analysis of the devices. GY and FT were responsible to draft the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yue, G., Cheng, R., Gao, X. et al. Synthesis of MoIn2S4@CNTs Composite Counter Electrode for Dye-Sensitized Solar Cells. Nanoscale Res Lett 15, 179 (2020). https://doi.org/10.1186/s11671-020-03410-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11671-020-03410-0