Abstract
Delivering drugs to the brain has always remained a challenge for the research community and physicians. The blood–brain barrier (BBB) acts as a major hurdle for delivering drugs to specific parts of the brain and the central nervous system. It is physiologically comprised of complex network of capillaries to protect the brain from any invasive agents or foreign particles. Therefore, there is an absolute need for understanding of the BBB for successful therapeutic interventions. Recent research indicates the strong emergence of zebrafish as a model for assessing the permeability of the BBB, which is highly conserved in its structure and function between the zebrafish and mammals. The zebrafish model system offers a plethora of advantages including easy maintenance, high fecundity and transparency of embryos and larvae. Therefore, it has the potential to be developed as a model for analysing and elucidating the permeability of BBB to novel permeation technologies with neurospecificity. Nanotechnology has now become a focus area within the industrial and research community for delivering drugs to the brain. Nanoparticles are being developed with increased efficiency and accuracy for overcoming the BBB and delivering neurospecific drugs to the brain. The zebrafish stands as an excellent model system to assess nanoparticle biocompatibility and toxicity. Hence, the zebrafish model is indispensable for the discovery or development of novel technologies for neurospecific drug delivery and potential therapies for brain diseases.
Similar content being viewed by others
Introduction
Drug delivery refers to the method of transferring compounds into the body for therapeutic purpose. The compounds are mainly pharmaceutical in nature and targeted against a particular disease condition to a particular cell population in vivo. The term drug delivery encompasses two main ideas: form of dosage and route of administration [1]. Proper drug delivery ensures efficient drug activity by regulating the following: drug release, absorption by cells and correct distribution within the system [2]. Some common drug delivery routes include enteral (gastrointestinal tract), parenteral (via injections), inhalation (olfactory mediated), transdermal (via dermis), topical (through skin) and oral routes (via oesophagus) [3]. Delivering a drug is critical and of major significance in the field of therapeutics. The chosen method must be most effective and also least toxic to the system [4]. The problem becomes even bigger when the organ in question is the brain. Delivering drugs to the brain has been a struggle among researchers for over decades now [5, 6]. Innumerable technologies and ideas have been used for the development of an effective technique [7, 8]. Yet, success doesn’t seem too near. The biggest hurdle in this struggle is the ability to cross the blood–brain barrier (BBB). The BBB is a physiological barrier to protect our brains from compounds being transferred from blood to the brain [9]. The natural makeup of the barrier allows only very small molecules in the blood stream to have access into the brain [10]. Molecules with small molecular weights < 400 Da and those which are lipid soluble have the ability to penetrate the brain [11]. Neurospecific drugs must meet these parameters for effective drug delivery across the BBB. At present, most of the drugs developed to target the brain are unsuccessful in crossing the BBB [9, 12, 13]. Diseases of the central nervous system are some of the most prevalent diseases affecting several people in all stages of life. However, these diseases still remain the least treated [14]. There is an immediate need for novel neurospecific drug delivery technologies since the success rates of existing drugs targeted to the brain is extremely low. Apart from the restricted permeability of the BBB, the complexity of the brain and the side effects caused by existing drug delivery technologies need to be taken care of as well [15]. Absence of an absolute method for efficient delivery of neurospecific drugs has hindered effective drug development in this field. The research community has explored various avenues for delivering safe and targeted drugs to the brain. Macromolecules to nanoparticles are being explored to ensure maximum effectiveness [16].
Nanotechnology has increasingly acquired the interest of the scientific community by its booming impact on research on brain drug delivery [17]. With the growth in nanotechnology, there has been a simultaneous expansion of the nanotoxicology sector. The toxicity assessment of the nanoparticles plays a pivotal role in analysing the impact of the nanoparticles to the individual species and the environment at large [18]. Recent years have seen the application of zebrafish as a prototype for toxicity studies [19]. The zebrafish has been used extensively for studies on experimental biology and is now evolving as a robust model system to study nanotoxicity [20]. In terms of model system for nanotoxicity, the zebrafish offers several advantages. It is highly economical to use as an experimental animal and easy to maintain. It has high fecundity rate making them easily available and helping to understand the vertebrate physiology in an easier way [21]. However, the use of zebrafish as a model system has its limitations too. First and foremost, the nervous system of the zebrafish may not be as complicated and developed as that in humans; the rodent and murine nervous systems are comparatively better developed and can be used to study the complex human brain diseases; however, they are not identical to that of humans [22]. Secondly, the zebrafish lacks some organ systems found in humans like the lungs, prostate and the mammary glands; also, diseases caused by genes absent in the zebrafish cannot be studied [23]. However, the zebrafish shares 70% genomic similarity with the human genome and 84% homology with human disease causing genes which makes it highly suitable to mimic human disease pathology [24]. The adult zebrafish had previously been postulated to be devoid of the liver macrophage; the Kupffer cells were regarded to be present only transiently in the early embryonic stage and absent or sparse in later stages of development [25,26,27]. However, recent work has shown the hematopoietic origin of the Kupffer cells and their persistence even in the adult zebrafish liver making zebrafish adept for research on Kupffer cells as well [28, 29]. Further, the higher vertebrate models are expected to mimic the complicated human pathologies to greater accuracy than the zebrafish. Recently, a debate has commenced over the reliance on data available from animal models and their extrapolation to humans [30]. This points out to the fact that any animal model whatsoever has its own limitations when applied to clinical studies [30, 31].
This review discusses the most recent studies on nanotechnology-mediated drug delivery specifically to the brain using the zebrafish as a model system. It summarizes the hurdles of the BBB and the various nanodrug optimizations, their toxicity evaluation and impact as use for therapeutics in neurodegenerative diseases using both zebrafish embryos and adults. Finally, the review highlights the advantages and disadvantages of the zebrafish model for neurospecific drug delivery and brings to light the immense scope it holds for future translational research.
Blood–Brain Barrier: The Main Obstacle in Neurospecific Drug Delivery
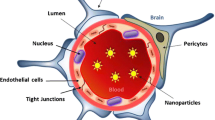
The BBB ensures restricted entry of substances into the brain, hence acting as a diffusion barrier helping to maintain normal brain homeostasis [32]. Several cells are involved in making up the composite structure of the BBB [33]. Pericytes, astrocytes and neurons comprise the cellular components, while the endothelial cells, tight junctions and basal membrane together constitute the BBB [34]. Lack of fenestrations in the endothelial cells in the brain ensures no diffusion of small molecules across their surface. Even water-soluble substances are hindered from entering the brain by the presence of inter endothelial junctions like tight junctions, adherens junctions and gap junctions, linking the endothelial cells [35]. These endothelial cells are in turn surrounded by the pericytes, astrocytes and basal membrane which complete the structure of the BBB [36]. The adherens junctions and tight junctions regulate the permeability of the endothelial cell layer. Gap junctions comprise of connexin molecules, and they control the communication between endothelial cells [37]. Molecules can cross the BBB via two pathways: the paracellular pathway or the transcellular pathway [38]. In the paracellular pathway, the ions and molecules pass the BBB by diffusing passively in between the cells using a concentration gradient [39]. The transcellular pathway employs the use of various mechanisms like transcytosis or receptor-mediated transport for passage of molecules through the cells [40]. Several parameters influence the permeability of the BBB. Molecular weight, charge on the surface, surface activity, solubility of the molecule and relative size of the molecule impact the BBB permeability [41].
Blood–Brain Barrier: Modern Technologies for Drug Delivery
The blood–brain barrier (BBB) in a healthy brain mainly operates as a diffusion barrier to protect normal brain functions. It prevents most of the compounds from being transferred from the blood to the brain. The stringent BBB allows only very small molecules to enter the brain; however, it is observed to be disrupted in disease conditions.
Why Nanoparticles Are a Current Choice for Neurospecific Drug Delivery
The technique of engineering and synthesizing materials at the molecular level is referred to as nanotechnology. The National Nanotechnology Institute defines nanotechnology as any material which exists in at least one dimension and ranges in size between 1 and 100 nm (Fig. 1). The last decade has seen a boom in the field of nanotechnology and its applications in the biomedical field. Nanotechnology-based drug delivery is believed to have stirred up the entire biotechnology and pharmaceutical industries and bring a profound change in this field in the coming years [42,43,44,45,46,47]. The application of nanotechnology promises several advantages in targeted drug delivery. These include the ability to deliver drugs (a) of less water solubility to their respective target site, (b) of two or more types for achieving combinatorial therapy, (c) targeted delivery at the specific site of action, (d) transport of drugs across tight barriers, i.e. blood–brain barrier, (e) visualization opportunities for better understanding and analysis of drug activity [48] and (f) real-time tracking facility for achieving perfect efficacy in mode of drug activity [44]. Thus, nanotechnology technique holds tremendous potential for neurospecific therapeutics.
Characteristics of neurospecific drugs. The BBB is typically made up of the tight junctions in the endothelial cells surrounded by the astrocytes, pericytes and neurons. Neurospecific molecules should possess specific characteristics to be able to cross the blood–brain barrier (BBB). Preferred characteristics are: very small size with a diameter of less than 100 nm, low molecular weight preferably less than 400 Da, should be positively charged, spherical in shape and lipid solubility
Zebrafish as a Model for Neurospecific Drug Delivery
The Danio rerio (zebrafish) is a demonstrated vertebrate model for exploring development studies and the study of degenerative diseases [49,50,51,52]. It can be modelled for far ranging analyses, from fundamental and toxicological analysis to pre-clinical studies [53,54,55]. Of the several advantages offered by the zebrafish, its cost-effective maintenance, easy testing with simple housing requirements and a large clutch size are highly suited for high throughput testing [56]. High fecundity is a distinctive feature which further accentuates use of this model system [24, 57]. The organ systems of zebrafish are highly conserved to that of higher vertebrates [58].
The zebrafish embryos have external development and are completely transparent as a result of which they can be extensively visually studied. Thus, they are an excellent tool for screening analyses using agents that disrupt normal growth, development and cell cycle [59]. They display thorough development patterns ranging from epiboly to final development of key structures [60, 61]. Zebrafish are now used extensively for neuropsychiatric research and various studies to analyse developmental toxicity in nanoparticle-mediated drug delivery. Exposure of zebrafish to gold nanoparticles disrupted normal eye development and pigmentation as observed via a simple light microscope [62, 63]. Administering gold nanoparticles to zebrafish resulted in genotoxic effects and serious alterations in their genome constitution [64]. The dose- and time-dependent toxicity of silica NPs was determined by analysing its impact on the cardiovascular system [65, 66] and on the mortality rates [67]. It was also found that chitosan NPs have higher compatibility as compared to normal chitosan [68].
It is absolutely vital that nanoparticles used for clinical interventions must be biodegradable and non-toxic. Nanoparticles have great potential in the field of targeted drug delivery and translational research. The use of nanoparticles has been applied to an increasingly large number of fields including in vivo applications. This wide increase in the use of the nanoparticles implicates the lurking danger of excessive exposure of these nanocarriers to humans. Toxicity studies of the nanoparticles are an indispensable part of nanotechnology. Studies focusing on nanoparticle interactions at the cellular and molecular levels must be undertaken to analyse the toxicity before they can be clinically used. Table 1 summarizes the neurotoxicity studies of diverse nanocarriers employed for brain targeted drug delivery using zebrafish. Nanoparticle toxicity involves analysing the toxicity, permeability, rate of mortality, induced teratogenicity, immune reactions and genomic toxicity.
Zebrafish is extensively used as a model system to evaluate nanoparticle toxicity and biocompatibility [111,112,113], and it holds great potential as a model for studying neurotoxicity and high throughput screening of nanoparticles [114,115,116,117]. No model other than the zebrafish is so aptly suited for such analyses. This model system can be used to study, analyse and manage the risks arising from toxicity of nanomaterials. The information gained will be helpful in formulating specific guidelines, framing protective measures and quality controls while working with nanotechnology-related products [118, 119].
Insights on Nanoparticles-Mediated Drug Delivery Using Zebrafish Embryos
In order to use nanoparticles for targeting the brain, a prior knowledge on their effects in vivo is essential. The zebrafish model is best suited for this purpose. Recent studies have been conducted using nanoparticles to gain vital insights into hatching of zebrafish larvae. Use of TiO2 nanoparticles induces early hatching in the larvae in a dose-dependent manner [120]. Chen et al. suggest that TiO2 nanoparticles have an impact on larval swimming behaviour affecting both velocity and the level of activity [121]. On the other hand, Ong et al. reported complete inhibition of hatching and embryonic death of larvae upon exposure to nanoparticles. They further added that the cause of death of embryos is the physical interaction of the nanoparticles with the embryos rather than the effects of the physico-chemical properties of the nanoparticles [122]. Disruption of the thyroid endocrine system in the zebrafish larvae has also been observed when they are exposed to TiO2 nanoparticles [123]. Accumulation of lead has been attributed to be the cause of this adverse effect. TiO2 nanoparticles have also been reported to significantly activate levels of expression of BDNF, C-fos and C-jun. Conversely, it was also found to have an inhibitory effect on genes such as p38, NGF and CRE resulting in the brain damage of zebrafish [124]. TiO2 nanoparticles have also been shown to have adverse effects on the reproductive potential of the fish causing 9.5% reduction in the number of the eggs released [125]. Vogt et al. further reported the chemical toxicity of the small molecule BCI when added to zebrafish embryos 24–48 h post-fertilization [126]. Ali and Legler et al. showed nonylphenol nanoparticle-induced malformations in the embryos even at low dose [127]. Usenko et al. evaluated carbon fullerene [C60, C70, and C60(OH)24]-induced toxicity using zebrafish embryos [128], while Daroczi et al. enumerated the protective potential of the same nanomaterial from ionizing radiation [129]. Neuroprotective effect of C60 fullerene derivative, dendrofullerene nanoparticle (DF-1), in the zebrafish embryos has also been reported by assessing its toxicity [129]. Administration of silica nanoparticles to the fish embryos resulted in enhanced mortality [67], while ZnO nanoparticles increased mortality and also caused skin ulceration with delay in hatching [82]. The impact of exposure of waterborne nanoparticles on genes which regulate the immune system was first reported by Brun et al. [130]. This study highlights the importance of molecular responses as indicators of biological toxicity. Zebrafish embryos engrafted with cancer cells and subjected to polymersome nanoparticle have been imaged real time to understand nanoparticle toxicity and treatment strategy [131].
Interestingly, bio-imaging using zebrafish embryos of various developmental stages revealed the toxic effects of sodium cholate enclustered Ag nanoparticles [132, 133]. This study holds immense importance [134] as it shows that toxicity arising from Ag nanoparticles affects the gills and lamelli development in the fish. This inhibitory effect is mainly caused by interaction of Ag ions in the gills where they block the Na + /K + ATPase activity [135, 136]. Further, it is reported that Cu nanoparticles have a similar inhibitory effect on the growth of gills in the fish [76]. Use of copper nanoparticles in the larvae led to malformation and delayed hatching [69, 76]. Application of gold nanoparticles had no toxic effect on the larvae [69], while silver nanoparticle affected development [137]. Nanoparticles made of zinc, magnesium, iron, copper and nickel had no toxicity on the adults, but in the larvae, delayed hatching has been observed [78, 79, 81, 82, 138]. Nanoparticles of organic compound fullerene have also been shown to be nontoxic to larvae at concentrations below 200 mg/L [139]. Furthermore, it was also shown that nanoparticles of chitosan were far more effective and non-toxic as compared to the usual chitosan particles [68].
Metal oxide nanoparticles like TiO2 have been reported to induce some developmental malformations in the zebrafish larvae [120], while some report that it is completely non-toxic [140, 141]. The crucial parameter here is the dosage as well as time of exposure. Higher doses of the TiO2 NPs prove to be fatal for the larvae with accumulations of the NP in the gill, heart, liver and brain [141, 142]. Genotoxic effects are also a result of exposure to high doses of TiO2 to the fish [143]. Chronic exposure to lower concentrations (< 4 mg/L) of TiO2 NPs leads to lower toxicity and a higher mortality rate [142]. Another important feature of a nanoparticle to be taken into concern is the shape of the nanoparticle and the proteins on its surface. Grain-column hexagonal crystals of ZnO NPs impacted the zebrafish cell cycle [144], whereas ZnO NPs which were leaf-shaped and coated with polymer displayed higher biocompatibility as compared to the spherical NPs [122]. Furthermore, it has been shown that nanosticks are more toxic than spheres and cuboidal nanoparticles [145]. Iron NPs lead to severe deformities in the larvae [146] and genotoxic effects in the adults [134], whereas metals like nickel, cobalt and aluminium NPs are proved to be relatively inert [82, 147].
Keeping in view the increased devastation caused by plastic in today’s world, Pitt et al. showed its impact on the zebrafish. They observed that the developing zebrafish are highly susceptible to the nanoplastic available in the aquatic ecosystems. These nanoparticles can penetrate the chorion and have dismal impact on their physiology and behavioural responses [148]. This study goes on to elucidate the nuisance created by plastic to the underwater world which in turn impacts human civilisation. Research suggests that very small nanoparticles which have high surface area/volume ratios are highly capable of absorbing pollutants from the environment. The use of polystyrene nanoplastic beads in cosmetic products has been studied for their developmental toxicity and impact on zebrafish embryos [149]. Another study on polystyrene nanoplastic of less than 20 nm size has shown that it accumulates in the brain of the embryos [150].
Insights Revealed by Nanoparticle Studies on Adult Zebrafish
A relatively extensive repertoire of research has been performed on the effects of nanoparticles on adult zebrafish. It acts as a valuable source of information on the use of nanoparticles in vertebrates. Truong et al. evaluated behavioural abnormality arising in 122 dpf embryos from exposure to gold nanoparticles [151]. Drug delivery to skin has also been accomplished by administering nanoparticles to the zebrafish. Researchers have shown that the Ag-BSA nanoparticles enter the skin by endocytosis where they accumulate and cause skin abnormalities [63]. Delivering drugs via nanoparticles have also been used to induce stress conditions in the zebrafish to act as potential models for drug discovery [152]. Some nanoparticles have been shown to induce asthma, apoptosis and enhanced immune response in the fish making it possible to use them for immunotoxicological studies [153,154,155,156]. Zebrafish model has been extensively studied for drug induced cardiotoxicity [157, 158]. The heart of a zebrafish exhibits several similar functional characteristics as that of a human heart including the pharmacologic drug responses [159,160,161,162]. The zebrafish heart is the first to develop at 22 hpf, while the entire cardiovascular system is ready by 48 hpf [163]. The zebrafish embryos have been visualized to study drug effects on heart rate, rhythmicity, contractility and circulation. Several visual assays have been performed using the zebrafish to help elaborate on cardiac health. A QT interval is one of the parameters on which most of the cardiac drugs are based on. The QT interval is the time gap between a Q and a T wave in the heart’s electrical cycle. A number of drugs have been assessed for their effect on QT interval (duration of ventricular action potential) using zebrafish [164,165,166]. One of the studies reported that drug causing prolongation of QT interval in humans actually leads to bradycardia and blocks auricular ventricular conduction [160]. The zebrafish liver forms by 48 hpf and becomes fully functional by 72 hpf; this model system is widely used to study liver based drug delivery. Studies in this field have revealed that the response exhibited by the zebrafish in hepatic toxicity is similar to that exhibited by the higher vertebrates [167]. The zebrafish have been used to characterize the orthologs of cytochrome P450, CYP3A and CYP3A65 [168, 169]. Further assessments have been performed to elaborate on the effect of drug on CYP3A4, CYP2D6 and CYP3A65 [170]. Neuroprotective effects of hesperetin nanoformulations have been studied in a traumatic brain injury model of zebrafish [171].
Zebrafish Offers a Complete Pathological Study Model for Neurospecific Drug Delivery
When delivering drug to the brain, several adverse effects can take place. The zebrafish model offers the advantage of studying these in detail and hence provides a suitable technique of drug delivery to the brain [172]. Teratogenicity: Any kind of abnormal teratogenic growth or development can be easily assessed by observing the transparent zebrafish embryos [59]. Of the key perturbations that can be observed during teratoma formation are the pigmentation of the eye [67], mortality rates [65], changes in the cardiovascular system [68] and effects on hatching [115]. Immunotoxicity: Research has been conducted on the immunological reactions that arise in the zebrafish in response to drugs or nanoparticles. This leads to accumulation of neutrophils and macrophages [173]. The use of gold nanoparticles has been reported to disrupt inflammatory immune responses [174], while on the other hand silver nanoparticles have been shown to induce inflammatory responses [175]. Genotoxicity: Changes occurring at the DNA level can be observed by real-time PCR [143] and other comet assays [134]. Recent research on carbon-based NPs have attracted increased attention recently [176] mainly because of their low toxicity [177]. Carbon NPs are used in various forms in zebrafish which include fullerenes [128], carbon nanoparticles, carbon nanotubes (CNT) [178], graphene QDs [179] and carbon QDs (C-dots) [180]. Allotropes of carbon such as fullerenes have also been used as NPs since their discovery in 1985. They have been used extensively for drug delivery applications [181, 182]. Studies in zebrafish revealed that toxicity of fullerene NPs is dependent on the charge on its surface. Positively charged fullerenes were more toxic as compared to the negatively charged fullerenes [128]. Research shows that water soluble fullerenes have the capacity to protect against cell death by acting as free radical scavengers [129, 183]. Recent research has been done in zebrafish with nano-onions which are multi-shell fullerene structures. They exhibit low toxicity and good bio compatibility in the zebrafish larvae [184]. Carbon nanotubes (CNTs) possess distinct physico-chemical characteristics for which they are an attractive mode of drug delivery for researchers [176, 185, 186]. Efficiency of CNTs depends on their length and the nature of their walls, whether single or multi-walled. Reports suggest that single- or multi-walled pristine CNTs have minimal impact on the growth and development of the zebrafish larvae [187]. Variations in the length of the CNTs may lead to changes at the molecular level with longer CNTs being more cytotoxic [188]. Adult zebrafish when exposed to multi-walled CNTs have shown to exhibit toxicity including inflammatory gills [189] and accumulation of the CNTs in the brain and gonads [105, 190]. Another form of carbon-based NPs are the quantum dots (QDs) and graphene quantum dots (GQDs). The typical feature of the QDs is quasi-spherical carbon structures with a diameter of less than 10 nm [191] and that of GQDs is less than 30 nm [192, 193]. An additional feature of the QDs includes their unique photostability which enables it to combine with the fluorophores thus opening up a score of bioimaging possibilities [194]. QDs exhibit least toxicity as they are predominantly composed of inert carbon molecules [195]. Hence, a combination of fluoroluminiscence and low toxicity properties makes it a very attractive tool for drug delivery [195,196,197].
Nanoparticles Focused for Delivering Drug to the Brain
With the background knowledge on action of nanoparticles on the physiology of zebrafish, researchers are now trying to deliver drugs to the brain via nanotechnology using the zebrafish models Table 2. Qian et al. have reported polymer nanoparticles conjugated with tags of phenylboronic acid on their surface which helps detect fluorescence for the neurotransmitter dopamine using zebrafish larvae [91]. This finding paves the way for theranostics of dopamine related diseases. However, a recent report elaborated on the toxicity of the gold nanoparticle as compared to ionic gold in zebrafish that were subjected to spiked sediment [64]. They reported that the nanoparticle altered neurotransmission in the zebrafish brain as it had an effect on the acetylcholine esterase activity. In an interesting work Sivaji et al. [198] aimed to deliver donepezil, a well-established drug for Alzheimer’s disease, through functionalized poly N-isopropyl acrylamide nanogels PNIPAM nanogel to the brain. They reported the gel could overcome the BBB and also showed sustained drug release using zebrafish model. This study therefore brings to the fore development of neurospecific nanogel for targeted drug delivery to the brain. The same group further reported synthesis of colloidal gold nanoparticle functionalized with polysorbate 80 and polyethylene glycol, with capabilities to overcome the blood–brain barrier for therapeutic purposes [199]. In this study they synthesized and validated a biocompatible nanocarrier with abilities to cross the blood–brain barrier and efficiently deliver neurospecific drugs.
Translational Approach of Neurospecific Nanoparticles: Zebrafish to Humans
A variety of model organisms have been employed till date to investigate human diseases. While chimpanzees and monkeys have a high degree of similarity with humans, mice and rats have been used extensively over the past few decades. Research using zebrafish models to study various human diseases is now on the increase [31]. Various state-of-the-art technologies have been analysed and evaluated using the zebrafish model. In this context, nanodiamonds (ND) which refer to a newer class of nanoparticles belonging to the carbon family are being explored in the latest techniques for drug delivery across the BBB [200, 201]. They possess outstanding optical properties, malleability of surface structures and mechanical properties which are pertinent for targeted drug delivery. The zebrafish has proved to be an apt model system to study the fluorescent nanodiamonds (FND) in detail. Chang et al. have studied the photostability and non-toxicity of FNDs by single particle tracking using zebrafish yolk cells [202]. Further, evaluation of ND to facilitate their application as nanolabels has been performed using laser confocal microscopy and real-time fluorescence tagging in zebrafish [203]. Zebrafish model can hence be explored to assess the potential of NDs as nanolabelling systems to deliver neurospecific drugs. The use of zebrafish is validated by its high genetic and systems similarity with that of humans. Regenerative ability of zebrafish is also a very interesting aspect of its physiology which has made it an important model organism to study neurodegenerative diseases. Recent studies have identified pivotal insights into brain drug delivery mechanism using zebrafish models of neurodegenerative diseases. Recent research conducted regarding drug delivery in the brain using the zebrafish model has revealed pivotal insights about the dynamics of this mechanism. The only drawback withholding accelerated research in this arena is the lack of established protocols to validate the studies. However, it is only a matter of time when such protocols are developed through ongoing research in this field. A great deal of scope still exists for further research on the following focus areas.
-
Admixture of nanoparticles along with two or more drugs to provide better holistic treatment
-
Analysis of fullerenes, nano-onions and nanodiamonds in neurodegenerative diseases
-
Understanding the biocompatibility of the newer nanoparticles and their brain-penetrating ability.
All the above-mentioned focus areas can be easily assessed using zebrafish model systems. The zebrafish model, therefore, holds great promise for development and evaluation of novel techniques for targeted drug delivery within the brain for translational analysis (Fig. 2). This could open up exciting new vistas for medical intervention to develop therapeutic strategies to treat neurodegenerative diseases.
Schematic representation of zebrafish model for delivering drugs encapsulated in nanoparticles to the brain. This method ensures efficient delivery of drugs across the blood–brain barrier (BBB). Several nanoparticles possess the potential to treat a variety of neurodegenerative diseases like Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), amyotrophic lateral sclerosis (ALS) and motor neuron diseases (MND)
Future Research Directions
The last decade witnessed a surge in the use of nanotechnology for brain drug delivery unfolding several exciting new strategies in this arena [16, 17, 204, 205]. However, problems like toxicity, immunogenicity and efficient drug delivery still persist and have restrained the research community from achieving their ultimate goal [206,207,208,209]. Future research prospects for neurospecific drug delivery therefore involve overcoming the existing challenges in this field. Research on nanomaterial toxicity and side effects should be extensive, accurate and always preceed the in vivo implementation of any new nanocarrier formulation. Proper comprehensive analysis of the nano-bio-interactions is absolutely essential for developing strategies for neurospecific drug delivery [210]. Newer imaging techniques should be adopted to broaden the understanding of bio distribution and pharmacokinetics of the delivered drug. Complete knowledge on the bio availability and clearance of the drug is indispensable for achieving the translation from bench side to bed side. Zebrafish, long considered as a “gold standard” for studying several developmental and metabolic diseases, is highly prospective for studies on nanodrug delivery. The transparent embryonic development with the ability to facilitate large-scale drug screening in a vertebrate model among other innumerable key attributes of the zebrafish holds promise for overcoming these roadblocks. The use of this robust model system therefore has immense potential for further research in nanotherapeutics to achieve safe and successful neurospecific drug delivery.
Conclusion
The BBB poses as the main obstacle in delivering drugs to the brain. The physiological function of the BBB is to protect the brain from foreign substances and in doing so it acts as a hurdle even for therapeutic purpose. The current need of the hour is a strategy in drug delivery which is able to overcome the BBB. Only then can effective treatments for brain specific diseases be possible. Recent focus on nanotechnology-based approaches for drug delivery across the BBB seems to have promising prospects for the field of neurospecific drug delivery in the future. Research towards this end is ongoing using a variety of nanoparticles like liposomes, dendrimers, micelles and carbon nanotubes as nanocarriers and nanogels. The zebrafish model is a favourite when it comes to nanotechnology-based toxicity studies and neurospecific drug delivery. Further research on nanotechnology using this model is needed for newer insights which can lead to possible breakthroughs in discovery in neurospecific drug delivery.
Availability of data and materials
Not applicable.
Abbreviations
- BBB:
-
Blood–brain barrier
- NPs:
-
Nanoparticles
- Au:
-
Gold
- Ag:
-
Silver
- Cu:
-
Copper
- Cd:
-
Cadmium
- CuO:
-
Copper oxide
- MgO:
-
Magnesium oxide
- NiO:
-
Nickel oxide
- ZnO:
-
Zinc oxide
- MPs:
-
Microplastics
- MOFs:
-
Metal organic frameworks
- CNTs:
-
Carbon nanotubes
- TiO2 :
-
Titanium dioxide
- QDs:
-
Quantum dots
- PCR:
-
Polymerase chain reaction
- GQDs:
-
Graphene quantum dots
- PNIPAM:
-
Poly N-isopropyl acrylamide
- NDs:
-
Nanodiamonds
- FND:
-
Fluorescent nanodiamonds
- AD:
-
Disease
- PD:
-
Parkinson’s disease
- HD:
-
Huntington’s disease
- ALS:
-
Amyotrophic lateral sclerosis
- MND:
-
Motor neuron diseases
References
Lavik EB, Kuppermann BD, Humayun MS (2013) Chapter 38—Drug delivery. In: Ryan SJ, Sadda SR, Hinton DR, Schachat AP, Sadda SR, Wilkinson CP et al (eds) Retina, 5th edn. W.B. Saunders, London, pp 734–745
Tiwari G, Tiwari R, Sriwastawa B, Bhati L, Pandey S, Pandey P et al (2012) Drug delivery systems: an updated review. Int J Pharm Investig 2(1):2–11
Hirota J, Shimizu S (2012) Chapter 5.2—Routes of administration. In: Hedrich HJ (ed) The laboratory mouse, 2nd edn. Academic Press, Boston, pp 709–725
Li C, Wang J, Wang Y, Gao H, Wei G, Huang Y et al (2019) Recent progress in drug delivery. Acta Pharm Sin B 9(6):1145–1162
Dong X (2018) Current strategies for brain drug delivery. Theranostics 8(6):1481–1493
Pardridge WM (2006) Molecular Trojan horses for blood–brain barrier drug delivery. Curr Opin Pharmacol 6(5):494–500
Pardridge WM (2002) Drug and gene delivery to the brain: the vascular route. Neuron 36(4):555–558
Huwyler J, Wu D, Pardridge WM (1996) Brain drug delivery of small molecules using immunoliposomes. Proc Natl Acad Sci USA 93(24):14164–14169
Bhowmik A, Khan R, Ghosh MK (2015) Blood brain barrier: a challenge for effectual therapy of brain tumors. BioMed Res Int 2015:320941
Kadry H, Noorani B, Cucullo L (2020) A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 17(1):69
Blasi P, Giovagnoli S, Schoubben A, Ricci M, Rossi C (2007) Solid lipid nanoparticles for targeted brain drug delivery. Adv Drug Deliv Rev 59(6):454–477
Pardridge WM (2020) Blood–brain barrier and delivery of protein and gene therapeutics to brain. Front Aging Neurosci 11:373
Upadhyay RK (2014) Drug delivery systems, CNS protection, and the blood brain barrier. BioMed Res Int 2014:869269
Kiaei M (2013) New hopes and challenges for treatment of neurodegenerative disorders: great opportunities for young neuroscientists. Basic Clin Neurosci 4(1):3–4
Bors LA, Erdő F (2019) Overcoming the blood–brain. Barrier challenges and tricks for CNS drug delivery. Sci Pharm 87(1):6
Masserini M (2013) Nanoparticles for brain drug delivery. ISRN Biochem 2013:238428
Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, Acosta-Torres LS et al (2018) Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol 16(1):71
Khan I, Saeed K, Khan I (2019) Nanoparticles: properties, applications and toxicities. Arab J Chem 12(7):908–931
Caballero MV, Candiracci M (2018) Zebrafish as screening model for detecting toxicity and drugs efficacy. J Unexplor Med Data 3:4
Dumitrescu E, Wallace K, Andreescu S (2019) Nanotoxicity assessment using embryonic zebrafish. Methods Mol Biol 1894:331–343
Goldsmith JR, Jobin C (2012) Think small: zebrafish as a model system of human pathology. J Biomed Biotechnol 2012:817341
Langova V, Vales K, Horka P, Horacek J (2020) The role of zebrafish and laboratory rodents in schizophrenia research. Front Psychiatry 11:703
Lin C-Y, Chiang C-Y, Tsai H-J (2016) Zebrafish and Medaka: new model organisms for modern biomedical research. J Biomed Sci 23:19
Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M et al (2013) The zebrafish reference genome sequence and its relationship to the human genome. Nature 496(7446):498–503
Roxo-Rosa M, Jacinto R, Sampaio P, Lopes SS (2015) The zebrafish Kupffer’s vesicle as a model system for the molecular mechanisms by which the lack of Polycystin-2 leads to stimulation of CFTR. Biol Open 4(11):1356–1366
Essner JJ, Amack JD, Nyholm MK, Harris EB, Yost HJ (2005) Kupffer’s vesicle is a ciliated organ of asymmetry in the zebrafish embryo that initiates left–right development of the brain, heart and gut. Development 132(6):1247–1260
Shwartz A, Goessling W, Yin C (2019) Macrophages in zebrafish models of liver diseases. Front Immunol 10:2840
Yang L, Jiménez JA, Earley AM, Hamlin V, Kwon V, Dixon CT et al (2020) Drainage of inflammatory macromolecules from the brain to periphery targets the liver for macrophage infiltration. Elife 9:e58191
He S, Chen J, Jiang Y, Wu Y, Zhu L, Jin W et al (2018) Adult zebrafish Langerhans cells arise from hematopoietic stem/progenitor cells. Elife 7:e36131
Van Norman GA (2019) Limitations of animal studies for predicting toxicity in clinical trials: Is it time to rethink our current approach? JACC Basic Transl Sci 4(7):845–854
Akhtar A (2015) The flaws and human harms of animal experimentation. Camb Q Healthc Ethics 24(4):407–419
Abbott NJ, Rönnbäck L, Hansson E (2006) Astrocyte–endothelial interactions at the blood–brain barrier. Nat Rev Neurosci 7(1):41–53
Miner JJ, Diamond MS (2016) Mechanisms of restriction of viral neuroinvasion at the blood-brain barrier. Curr Opin Immunol 38:18–23
Correale J, Villa A (2009) Cellular elements of the blood–brain barrier. Neurochem Res 34(12):2067
Liu W-Y, Wang Z-B, Zhang L-C, Wei X, Li L (2012) Tight junction in blood–brain barrier: an overview of structure, regulation, and regulator substances. CNS Neurosci Ther 18(8):609–615
Gastfriend BD, Palecek SP, Shusta EV (2018) Modeling the blood–brain barrier: beyond the endothelial cells. Curr Opin Biomed Eng 5:6–12
Stamatovic SM, Keep RF, Andjelkovic AV (2008) Brain endothelial cell–cell junctions: how to “open” the blood brain barrier. Curr Neuropharmacol 6(3):179–192
Knowland D, Arac A, Sekiguchi Kohei J, Hsu M, Lutz Sarah E, Perrino J et al (2014) Stepwise recruitment of transcellular and paracellular pathways underlies blood–brain barrier breakdown in stroke. Neuron 82(3):603–617
O’Keeffe E, Campbell M (2016) Modulating the paracellular pathway at the blood–brain barrier: current and future approaches for drug delivery to the CNS. Drug Discov Today Technol 20:35–39
von Wedel-Parlow M, Schrot S, Lemmen J, Treeratanapiboon L, Wegener J, Galla H-J (2011) Neutrophils cross the BBB primarily on transcellular pathways: an in vitro study. Brain Res 1367:62–76
Almutairi MM, Gong C, Xu YG, Chang Y, Shi H (2016) Factors controlling permeability of the blood–brain barrier. Cell Mol Life Sci CMLS 73(1):57–77
Whitesides GM (2003) The “right” size in nanobiotechnology. Nat Biotechnol 21(10):1161–1165
LaVan DA, McGuire T, Langer R (2003) Small-scale systems for in vivo drug delivery. Nat Biotechnol 21(10):1184–1191
Ferrari M (2005) Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer 5(3):161–171
Farokhzad OC, Karp JM, Langer R (2006) Nanoparticle-aptamer bioconjugates for cancer targeting. Expert Opin Drug Deliv 3(3):311–324
Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC (2008) Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther 83(5):761–769
Langer R (1998) Drug delivery and targeting. Nature 392(6679 Suppl):5–10
Liong M, Lu J, Kovochich M, Xia T, Ruehm SG, Nel AE et al (2008) Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano 2(5):889–896
Saleem S, Kannan RR (2018) Zebrafish: an emerging real-time model system to study Alzheimer’s disease and neurospecific drug discovery. Cell Death Discov 4:45
Granato M, Nusslein-Volhard C (1996) Fishing for genes controlling development. Curr Opin Genet Dev 6(4):461–468
Haffter P, Granato M, Brand M, Mullins MC, Hammerschmidt M, Kane DA et al (1996) The identification of genes with unique and essential functions in the development of the zebrafish, Danio rerio. Development 123:1–36
Talbot WS, Hopkins N (2000) Zebrafish mutations and functional analysis of the vertebrate genome. Genes Dev 14(7):755–762
Strahle U, Scholz S, Geisler R, Greiner P, Hollert H, Rastegar S et al (2012) Zebrafish embryos as an alternative to animal experiments–a commentary on the definition of the onset of protected life stages in animal welfare regulations. Reprod Toxicol 33(2):128–132
Chakraborty C, Agoramoorthy G (2010) Why zebrafish? Riv Biol 103(1):25–27
White DT, Saxena MT, Mumm JS (2019) Let’s get small (and smaller): combining zebrafish and nanomedicine to advance neuroregenerative therapeutics. Adv Drug Deliv Rev 148:344–359
Dooley K, Zon LI (2000) Zebrafish: a model system for the study of human disease. Curr Opin Genet Dev 10(3):252–256
Kettleborough RN, Busch-Nentwich EM, Harvey SA, Dooley CM, de Bruijn E, van Eeden F et al (2013) A systematic genome-wide analysis of zebrafish protein-coding gene function. Nature 496(7446):494–497
Hsu CH, Wen ZH, Lin CS, Chakraborty C (2007) The zebrafish model: use in studying cellular mechanisms for a spectrum of clinical disease entities. Curr Neurovasc Res 4(2):111–120
Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF (1995) Stages of embryonic development of the zebrafish. Dev Dyn Off Publ Am Assoc Anat 203(3):253–310
He JH, Gao JM, Huang CJ, Li CQ (2014) Zebrafish models for assessing developmental and reproductive toxicity. Neurotoxicol Teratol 42:35–42
Nishimura Y, Inoue A, Sasagawa S, Koiwa J, Kawaguchi K, Kawase R et al (2016) Using zebrafish in systems toxicology for developmental toxicity testing. Congenit Anom 56(1):18–27
Kim KT, Zaikova T, Hutchison JE, Tanguay RL (2013) Gold nanoparticles disrupt zebrafish eye development and pigmentation. Toxicol Sci Off J Soc Toxicol 133(2):275–288
Durairaj B, Dhanabal M (2020) Zebrafish as a prodigious tool in neuropsychiatric research. J Basic Appl Zool 81(1):54
Dedeh A, Ciutat A, Treguer-Delapierre M, Bourdineaud JP (2015) Impact of gold nanoparticles on zebrafish exposed to a spiked sediment. Nanotoxicology 9(1):71–80
Paatero I, Casals E, Niemi R, Ozliseli E, Rosenholm JM, Sahlgren C (2017) Analyses in zebrafish embryos reveal that nanotoxicity profiles are dependent on surface-functionalization controlled penetrance of biological membranes. Sci Rep 7(1):8423
Geffroy B, Ladhar C, Cambier S, Treguer-Delapierre M, Brethes D, Bourdineaud JP (2012) Impact of dietary gold nanoparticles in zebrafish at very low contamination pressure: the role of size, concentration and exposure time. Nanotoxicology 6(2):144–160
Duan J, Yu Y, Shi H, Tian L, Guo C, Huang P et al (2013) Toxic effects of silica nanoparticles on zebrafish embryos and larvae. PLoS ONE 8(9):e74606
Wang Y, Zhou J, Liu L, Huang C, Zhou D, Fu L (2016) Characterization and toxicology evaluation of chitosan nanoparticles on the embryonic development of zebrafish, Danio rerio. Carbohydr Polym 141:204–210
Kovriznych JA, Sotnikova R, Zeljenkova D, Rollerova E, Szabova E, Wimmerova S (2013) Acute toxicity of 31 different nanoparticles to zebrafish (Danio rerio) tested in adulthood and in early life stages—comparative study. Interdiscip Toxicol 6(2):67–73
Kim K-T, Zaikova T, Hutchison JE, Tanguay RL (2013) Gold nanoparticles disrupt zebrafish eye development and pigmentation. Toxicol Sci 133(2):275–288
Browning LM, Lee KJ, Huang T, Nallathamby PD, Lowman JE, Xu X-HN (2009) Random walk of single gold nanoparticles in zebrafish embryos leading to stochastic toxic effects on embryonic developments. Nanoscale 1(1):138–152
Patibandla S, Zhang Y, Tohari AM, Gu P, Reilly J, Chen Y et al (2018) Comparative analysis of the toxicity of gold nanoparticles in zebrafish. J Appl Toxicol 38(8):1153–1161
Bai C, Tang M (2020) Toxicological study of metal and metal oxide nanoparticles in zebrafish. J Appl Toxicol 40(1):37–63
Cörek E, Rodgers G, Siegrist S, Einfalt T, Detampel P, Schlepütz CM et al (2020) Shedding light on metal-based nanoparticles in zebrafish by computed tomography with micrometer resolution. Small 16(31):2000746
Cáceres-Vélez PR, Fascineli ML, Sousa MH, Grisolia CK, Yate L, de Souza PEN et al (2018) Humic acid attenuation of silver nanoparticle toxicity by ion complexation and the formation of a Ag(3+) coating. J Hazard Mater 353:173–181
Griffitt RJ, Weil R, Hyndman KA, Denslow ND, Powers K, Taylor D et al (2007) Exposure to copper nanoparticles causes gill injury and acute lethality in zebrafish (Danio rerio). Environ Sci Technol 41(23):8178–8186
Zhang W, Lin K, Miao Y, Dong Q, Huang C, Wang H et al (2012) Toxicity assessment of zebrafish following exposure to CdTe QDs. J Hazard Mater 213–214:413–420
Kumari P, Panda PK, Jha E, Kumari K, Nisha K, Mallick MA et al (2017) Mechanistic insight to ROS and Apoptosis regulated cytotoxicity inferred by Green synthesized CuO nanoparticles from Calotropis gigantea to embryonic zebrafish. Sci Rep 7(1):16284
Ghobadian M, Nabiuni M, Parivar K, Fathi M, Pazooki J (2015) Toxic effects of magnesium oxide nanoparticles on early developmental and larval stages of zebrafish (Danio rerio). Ecotoxicol Environ Saf 122:260–267
Kovrižnych JA, Sotníková R, Zeljenková D, Rollerová E, Szabová E (2014) Long-term (30 days) toxicity of NiO nanoparticles for adult zebrafish Danio rerio. Interdiscip Toxicol 7(1):23–26
Wehmas LC, Anders C, Chess J, Punnoose A, Pereira CB, Greenwood JA et al (2015) Comparative metal oxide nanoparticle toxicity using embryonic zebrafish. Toxicol Rep 2:702–715
Zhu X, Zhu L, Duan Z, Qi R, Li Y, Lang Y (2008) Comparative toxicity of several metal oxide nanoparticle aqueous suspensions to Zebrafish (Danio rerio) early developmental stage. J Environ Sci Health Part A Toxic hazard Subst Environ Eng 43(3):278–284
Malhotra N, Chen J-R, Sarasamma S, Audira G, Siregar P, Liang S-T et al (2019) Ecotoxicity assessment of Fe(3)O(4) magnetic nanoparticle exposure in adult zebrafish at an environmental pertinent concentration by behavioral and biochemical testing. Nanomaterials (Basel) 9(6):873
de Oliveira GMT, Kist LW, Pereira TCB, Bortolotto JW, Paquete FL, de Oliveira EMN et al (2014) Transient modulation of acetylcholinesterase activity caused by exposure to dextran-coated iron oxide nanoparticles in brain of adult zebrafish. Comp Biochem Physiol Part C Toxicol Pharmacol 162:77–84
Zhu X, Tian S, Cai Z (2012) Toxicity assessment of iron oxide nanoparticles in zebrafish (Danio rerio) early life stages. PLoS ONE 7(9):e46286
Jia H-R, Zhu Y-X, Xu K-F, Pan G-Y, Liu X, Qiao Y et al (2019) Efficient cell surface labelling of live zebrafish embryos: wash-free fluorescence imaging for cellular dynamics tracking and nanotoxicity evaluation. Chem Sci 10(14):4062–4068
Kögel T, Bjorøy Ø, Toto B, Bienfait AM, Sanden M (2020) Micro- and nanoplastic toxicity on aquatic life: determining factors. Sci Total Environ 709:136050
Sarasamma S, Audira G, Siregar P, Malhotra N, Lai Y-H, Liang S-T et al (2020) Nanoplastics cause neurobehavioral impairments, reproductive and oxidative damages, and biomarker responses in zebrafish: throwing up alarms of wide spread health risk of exposure. Int J Mol Sci 21(4):1410
Zhao Y, Xiong S, Liu P, Liu W, Wang Q, Liu Y et al (2020) Polymeric nanoparticles-based brain delivery with improved therapeutic efficacy of Ginkgolide B in Parkinson’s disease. Int J Nanomed 15:10453–10467
Rabanel J-M, Piec P-A, Landri S, Patten SA, Ramassamy C (2020) Transport of PEGylated-PLA nanoparticles across a blood brain barrier model, entry into neuronal cells and in vivo brain bioavailability. J Controll Release 328:679–695
Qian C-G, Zhu S, Feng P-J, Chen Y-L, Yu J-C, Tang X et al (2015) Conjugated polymer nanoparticles for fluorescence imaging and sensing of neurotransmitter dopamine in living cells and the brains of zebrafish larvae. ACS Appl Mater Interfaces 7(33):18581–18589
Yang T, Martin P, Fogarty B, Brown A, Schurman K, Phipps R et al (2015) Exosome delivered anticancer drugs across the blood–brain barrier for brain cancer therapy in Danio rerio. Pharm Res 32(6):2003–2014
Yang T, Fogarty B, LaForge B, Aziz S, Pham T, Lai L et al (2017) Delivery of small interfering RNA to inhibit vascular endothelial growth factor in zebrafish using natural brain endothelia cell-secreted exosome nanovesicles for the treatment of brain cancer. AAPS J 19(2):475–486
Wu Z, Koh B, Lawrence LM, Kanamala M, Pool B, Svirskis D et al (2019) Liposome-mediated drug delivery in larval zebrafish to manipulate macrophage function. Zebrafish 16(2):171–181
Au-Linnerz T, Au-Kanamala M, Au-Astin JW, Au-Dalbeth N, Au-Wu Z, Au-Hall CJ (2020) Targeting drugs to larval zebrafish macrophages by injecting drug-loaded liposomes. JoVE 156:e60198
Yang L, Rojas AM, Shiau CE (2021) Liposomal clodronate-mediated macrophage depletion in the zebrafish model. Bio-Protoc 11(6):e3951
Dal N-JK, Kocere A, Wohlmann J, Van Herck S, Bauer TA, Resseguier J et al (2020) Zebrafish embryos allow prediction of nanoparticle circulation times in mice and facilitate quantification of nanoparticle-cell interactions. Small 16(5):1919
Sieber S, Grossen P, Uhl P, Detampel P, Mier W, Witzigmann D et al (2019) Zebrafish as a predictive screening model to assess macrophage clearance of liposomes in vivo. Nanomed Nanotechnol Biol Med 17:82–93
Wang YJ, Yuan B, Liu W, Li YH, Yuan XY (2020) The effect of nanoscale zirconium–porphyrin metal-organic framework on zebrafish embryonic neurodevelopment. Zhongguo Ying Yong Sheng Li Xue Za Zhi 36(6):662–667
Abramenko N, Deyko G, Abkhalimov E, Isaeva V, Pelgunova L, Krysanov E et al (2021) Acute toxicity of Cu-MOF nanoparticles (nanoHKUST-1) towards embryos and adult zebrafish. Int J Mol Sci 22(11):5568
Ruyra À, Yazdi A, Espín J, Carné-Sánchez A, Roher N, Lorenzo J et al (2015) Synthesis, culture medium stability, and in vitro and in vivo zebrafish embryo toxicity of metal–organic framework nanoparticles. Chem Eur J 21(6):2508–2518
da Rocha AM, Kist LW, Almeida EA, Silva DGH, Bonan CD, Altenhofen S et al (2019) Neurotoxicity in zebrafish exposed to carbon nanotubes: effects on neurotransmitters levels and antioxidant system. Comp Biochem Physiol Part C Toxicol Pharmacol 218:30–35
da Rocha AM, Ferreira JR, Barros DM, Pereira TCB, Bogo MR, Oliveira S et al (2013) Gene expression and biochemical responses in brain of zebrafish Danio rerio exposed to organic nanomaterials: carbon nanotubes (SWCNT) and fullerenol (C60(OH)18–22(OK4)). Comp Biochem Physiol Part A Mol Integr Physiol 165(4):460–467
Martinez CS, Igartúa DE, Czarnowski I, Feas DA, Alonso SD, Prieto MJ (2019) Biological response and developmental toxicity of zebrafish embryo and larvae exposed to multi-walled carbon nanotubes with different dimension. Heliyon 5(8):e02308
Li J, Ying G-G, Jones KC, Martin FL (2015) Real-world carbon nanoparticle exposures induce brain and gonadal alterations in zebrafish (Danio rerio) as determined by biospectroscopy techniques. Analyst 140(8):2687–2695
Ganesan R, Vasantha-Srinivasan P, Sadhasivam DR, Subramanian R, Vimalraj S, Suk KT (2021) Carbon nanotubes induce metabolomic profile disturbances in zebrafish: NMR-based metabolomics platform. Front Mol Biosci 8:632
Gorrochategui E, Li J, Fullwood NJ, Ying G-G, Tian M, Cui L et al (2016) Diet-sourced carbon-based nanoparticles induce lipid alterations in tissues of zebrafish (Danio rerio) with genomic hypermethylation changes in brain. Mutagenesis 32(1):91–103
Ren C, Hu X, Zhou Q (2018) Graphene oxide quantum dots reduce oxidative stress and inhibit neurotoxicity in vitro and in vivo through catalase-like activity and metabolic regulation. Adv Sci (Weinh) 5(5):1700595
Li S, Peng Z, Dallman J, Baker J, Othman AM, Blackwelder PL et al (2016) Crossing the blood–brain–barrier with transferrin conjugated carbon dots: a zebrafish model study. Colloids Surf B Biointerfaces 145:251–256
Rieger S, Kulkarni RP, Darcy D, Fraser SE, Köster RW (2005) Quantum dots are powerful multipurpose vital labeling agents in zebrafish embryos. Dev Dyn 234(3):670–681
Jia H-R, Zhu Y-X, Duan Q-Y, Chen Z, Wu F-G (2019) Nanomaterials meet zebrafish: toxicity evaluation and drug delivery applications. J Controll Release 311–312:301–318
Martinez CS, Igartúa DE, Calienni MN, Feas DA, Siri M, Montanari J et al (2017) Relation between biophysical properties of nanostructures and their toxicity on zebrafish. Biophys Rev 9(5):775–791
Pensado-López A, Fernández-Rey J, Reimunde P, Crecente-Campo J, Sánchez L, Torres Andón F (2021) Zebrafish models for the safety and therapeutic testing of nanoparticles with a focus on macrophages. Nanomaterials 11(7):174
Jagdale SC, Hude RU, Chabukswar AR (2020) Zebrafish: a laboratory model to evaluate nanoparticle toxicity. In: Siddhardha B, Dyavaiah M, Kasinathan K (eds) Model organisms to study biological activities and toxicity of nanoparticles. Springer, Singapore, pp 371–399
Chakraborty C, Sharma AR, Sharma G, Lee S-S (2016) Zebrafish: a complete animal model to enumerate the nanoparticle toxicity. J Nanobiotechnol 14(1):65
De Jimmy L, del María CC, Francisco M (2019) Toxicology of nanomaterials on zebrafish. Am J Eng Appl Sci 12(2):193–203
Haque E, Ward AC (2018) Zebrafish as a model to evaluate nanoparticle toxicity. Nanomaterials (Basel) 8(7):561
Kahru A, Dubourguier HC (2010) From ecotoxicology to nanoecotoxicology. Toxicology 269(2–3):105–119
Youtie J, Porter A, Shapira P, Tang L, Benn T (2011) The use of environmental, health and safety research in nanotechnology research. J Nanosci Nanotechnol 11(1):158–166
Samaee SM, Rabbani S, Jovanovic B, Mohajeri-Tehrani MR, Haghpanah V (2015) Efficacy of the hatching event in assessing the embryo toxicity of the nano-sized TiO(2) particles in zebrafish: a comparison between two different classes of hatching-derived variables. Ecotoxicol Environ Saf 116:121–128
Chen TH, Lin CY, Tseng MC (2011) Behavioral effects of titanium dioxide nanoparticles on larval zebrafish (Danio rerio). Mar Pollut Bull 63(5–12):303–308
Ong KJ, Zhao X, Thistle ME, Maccormack TJ, Clark RJ, Ma G et al (2014) Mechanistic insights into the effect of nanoparticles on zebrafish hatch. Nanotoxicology 8(3):295–304
Miao W, Zhu B, Xiao X, Li Y, Dirbaba NB, Zhou B et al (2015) Effects of titanium dioxide nanoparticles on lead bioconcentration and toxicity on thyroid endocrine system and neuronal development in zebrafish larvae. Aquat Toxicol 161:117–126
Sheng L, Wang L, Su M, Zhao X, Hu R, Yu X et al (2016) Mechanism of TiO2 nanoparticle-induced neurotoxicity in zebrafish (Danio rerio). Environ Toxicol 31(2):163–175
Wang J, Zhu X, Zhang X, Zhao Z, Liu H, George R et al (2011) Disruption of zebrafish (Danio rerio) reproduction upon chronic exposure to TiO(2) nanoparticles. Chemosphere 83(4):461–467
Vogt A, Codore H, Day BW, Hukriede NA, Tsang M (2010) Development of automated imaging and analysis for zebrafish chemical screens. J Vis Exp JoVE. https://doi.org/10.3791/1900
Chandrasekar G, Arner A, Kitambi SS, Dahlman-Wright K, Lendahl MA (2011) Developmental toxicity of the environmental pollutant 4-nonylphenol in zebrafish. Neurotoxicol Teratol 33(6):752–764
Usenko CY, Harper SL, Tanguay RL (2007) In vivo evaluation of carbon fullerene toxicity using embryonic zebrafish. Carbon 45(9):1891–1898
Daroczi B, Kari G, McAleer MF, Wolf JC, Rodeck U, Dicker AP (2006) In vivo radioprotection by the fullerene nanoparticle DF-1 as assessed in a zebrafish model. Clin Cancer Res Off J Am Assoc Cancer Res 12(23):7086–7091
Brun NR, Koch BEV, Varela M, Peijnenburg WJGM, Spaink HP, Vijver MG (2018) Nanoparticles induce dermal and intestinal innate immune system responses in zebrafish embryos. Environ Sci Nano 5(4):904–916
Kocere A, Resseguier J, Wohlmann J, Skjeldal FM, Khan S, Speth M et al (2020) Real-time imaging of polymersome nanoparticles in zebrafish embryos engrafted with melanoma cancer cells: localization, toxicity and treatment analysis. EBioMedicine 58:102902
Browning LM, Huang T, Xu XH (2013) Real-time in vivo imaging of size-dependent transport and toxicity of gold nanoparticles in zebrafish embryos using single nanoparticle plasmonic spectroscopy. Interface Focus 3(3):20120098
Chandirasekar S, Chandrasekaran C, Muthukumarasamyvel T, Sudhandiran G, Rajendiran N (2015) Sodium cholate-templated blue light-emitting Ag subnanoclusters: in vivo toxicity and imaging in zebrafish embryos. ACS Appl Mater Interfaces 7(3):1422–1430
Villacis RAR, Filho JS, Pina B, Azevedo RB, Pic-Taylor A, Mazzeu JF et al (2017) Integrated assessment of toxic effects of maghemite (gamma-Fe2O3) nanoparticles in zebrafish. Aquat Toxicol 191:219–225
Bury NR, Grosell M, Grover AK, Wood CM (1999) ATP-dependent silver transport across the basolateral membrane of rainbow trout gills. Toxicol Appl Pharmacol 159(1):1–8
Grosell M, Nielsen C, Bianchini A (2002) Sodium turnover rate determines sensitivity to acute copper and silver exposure in freshwater animals. Comp Biochem Physiol Toxicol Pharmacol CBP 133(1–2):287–303
Caceres-Velez PR, Fascineli ML, Sousa MH, Grisolia CK, Yate L, de Souza PEN et al (2018) Humic acid attenuation of silver nanoparticle toxicity by ion complexation and the formation of a Ag(3+) coating. J Hazard Mater 353:173–181
Kovriznych JA, Sotnikova R, Zeljenkova D, Rollerova E, Szabova E (2014) Long-term (30 days) toxicity of NiO nanoparticles for adult zebrafish Danio rerio. Interdiscip Toxicol 7(1):23–26
Zhu X, Zhu L, Li Y, Duan Z, Chen W, Alvarez PJ (2007) Developmental toxicity in zebrafish (Danio rerio) embryos after exposure to manufactured nanomaterials: buckminsterfullerene aggregates (nC60) and fullerol. Environ Toxicol Chem 26(5):976–979
Wang YJ, He ZZ, Fang YW, Xu Y, Chen YN, Wang GQ et al (2014) Effect of titanium dioxide nanoparticles on zebrafish embryos and developing retina. Int J Ophthalmol 7(6):917–923
Clemente Z, Castro VL, Moura MA, Jonsson CM, Fraceto LF (2014) Toxicity assessment of TiO(2) nanoparticles in zebrafish embryos under different exposure conditions. Aquat Toxicol 147:129–139
Chen J, Dong X, Xin Y, Zhao M (2011) Effects of titanium dioxide nano-particles on growth and some histological parameters of zebrafish (Danio rerio) after a long-term exposure. Aquat Toxicol 101(3–4):493–499
Rocco L, Santonastaso M, Mottola F, Costagliola D, Suero T, Pacifico S et al (2015) Genotoxicity assessment of TiO2 nanoparticles in the teleost Danio rerio. Ecotoxicol Environ Saf 113:223–230
Hou J, Liu H, Zhang S, Liu X, Hayat T, Alsaedi A et al (2019) Mechanism of toxic effects of nano-ZnO on cell cycle of zebrafish (Danio rerio). Chemosphere 229:206–213
Hua J, Vijver MG, Richardson MK, Ahmad F, Peijnenburg WJ (2014) Particle-specific toxic effects of differently shaped zinc oxide nanoparticles to zebrafish embryos (Danio rerio). Environ Toxicol Chem 33(12):2859–2868
Liu Y, Liu B, Feng D, Gao C, Wu M, He N et al (2012) A progressive approach on zebrafish toward sensitive evaluation of nanoparticles’ toxicity. Integr Biol Quant Biosci Nano Macro 4(3):285–291
Lin S, Zhao Y, Xia T, Meng H, Ji Z, Liu R et al (2011) High content screening in zebrafish speeds up hazard ranking of transition metal oxide nanoparticles. ACS Nano 5(9):7284–7295
Pitt JA, Kozal JS, Jayasundara N, Massarsky A, Trevisan R, Geitner N et al (2018) Uptake, tissue distribution, and toxicity of polystyrene nanoparticles in developing zebrafish (Danio rerio). Aquat Toxicol 194:185–194
Liu Y, Wang Y, Ling X, Yan Z, Wu D, Liu J et al (2021) Effects of nanoplastics and butyl methoxydibenzoylmethane on early zebrafish embryos identified by single-cell RNA sequencing. Environ Sci Technol 55(3):1885–1896
Sökmen TÖ, Sulukan E, Türkoğlu M, Baran A, Özkaraca M, Ceyhun SB (2020) Polystyrene nanoplastics (20 nm) are able to bioaccumulate and cause oxidative DNA damages in the brain tissue of zebrafish embryo (Danio rerio). NeuroToxicol 77:51–59
Truong L, Saili KS, Miller JM, Hutchison JE, Tanguay RL (2012) Persistent adult zebrafish behavioral deficits results from acute embryonic exposure to gold nanoparticles. Comp Biochem Physiol Toxicol Pharmacol CBP 155(2):269–274
Lankveld DP, Van Loveren H, Baken KA, Vandebriel RJ (2010) In vitro testing for direct immunotoxicity: state of the art. Methods Mol Biol 598:401–423
Di Gioacchino M, Petrarca C, Lazzarin F, Di Giampaolo L, Sabbioni E, Boscolo P et al (2011) Immunotoxicity of nanoparticles. Int J Immunopathol Pharmacol 24(1 Suppl):65S-71S
Jin Y, Zheng S, Fu Z (2011) Embryonic exposure to cypermethrin induces apoptosis and immunotoxicity in zebrafish (Danio rerio). Fish Shellfish Immunol 30(4–5):1049–1054
Zhuang S, Zhang Z, Zhang W, Bao L, Xu C, Zhang H (2015) Enantioselective developmental toxicity and immunotoxicity of pyraclofos toward zebrafish (Danio rerio). Aquat Toxicol 159:119–126
Xu H, Dong X, Zhang Z, Yang M, Wu X, Liu H et al (2015) Assessment of immunotoxicity of dibutyl phthalate using live zebrafish embryos. Fish Shellfish Immunol 45(2):286–292
Parng C, Seng WL, Semino C, McGrath P (2002) Zebrafish: a preclinical model for drug screening. Assay Drug Dev Technol 1(1 Pt 1):41–48
Parng C (2005) In vivo zebrafish assays for toxicity testing. Curr Opin Drug Discov Dev 8(1):100–106
Langheinrich U, Vacun G, Wagner T (2003) Zebrafish embryos express an orthologue of HERG and are sensitive toward a range of QT-prolonging drugs inducing severe arrhythmia. Toxicol Appl Pharmacol 193(3):370–382
Sedmera D, Reckova M, deAlmeida A, Sedmerova M, Biermann M, Volejnik J et al (2003) Functional and morphological evidence for a ventricular conduction system in zebrafish and Xenopus hearts. Am J Physiol Heart Circ Physiol 284(4):H1152–H1160
Milan DJ, Peterson TA, Ruskin JN, Peterson RT, MacRae CA (2003) Drugs that induce repolarization abnormalities cause bradycardia in zebrafish. Circulation 107(10):1355–1358
Milan DJ, Jones IL, Ellinor PT, MacRae CA (2006) In vivo recording of adult zebrafish electrocardiogram and assessment of drug-induced QT prolongation. Am J Physiol Heart Circ Physiol 291(1):H269–H273
Thisse C, Zon LI (2002) Organogenesis–heart and blood formation from the zebrafish point of view. Science 295(5554):457–462
Ackerman MJ (1998) The long QT syndrome: ion channel diseases of the heart. Mayo Clin Proc 73(3):250–269
Anderson ME, Al-Khatib SM, Roden DM, Califf RM, Duke Clinical Research Institute/American Heart Journal Expert Meeting on Repolarization C (2002) Cardiac repolarization: current knowledge, critical gaps, and new approaches to drug development and patient management. Am Heart J 144(5):769–781
Lawrence CL, Pollard CE, Hammond TG, Valentin JP (2005) Nonclinical proarrhythmia models: predicting Torsades de Pointes. J Pharmacol Toxicol Methods 52(1):46–59
Zhang C, Willett C, Fremgen T (2003) Zebrafish: an animal model for toxicological studies. Curr Protoc Toxicol 17:1–7
Kubota A, Bainy AC, Woodin BR, Goldstone JV, Stegeman JJ (2013) The cytochrome P450 2AA gene cluster in zebrafish (Danio rerio): expression of CYP2AA1 and CYP2AA2 and response to phenobarbital-type inducers. Toxicol Appl Pharmacol 272(1):172–179
Tseng HP, Hseu TH, Buhler DR, Wang WD, Hu CH (2005) Constitutive and xenobiotics-induced expression of a novel CYP3A gene from zebrafish larva. Toxicol Appl Pharmacol 205(3):247–258
Kocarek TA, Schuetz EG, Strom SC, Fisher RA, Guzelian PS (1995) Comparative analysis of cytochrome P4503A induction in primary cultures of rat, rabbit, and human hepatocytes. Drug Metab Dispos Biol Fate Chem 23(3):415–421
Paramita P, Sethu SN, Subhapradha N, Ragavan V, Ilangovan R, Balakrishnan A et al (2020) Neuro-protective effects of nano-formulated hesperetin in a traumatic brain injury model of Danio rerio. Drug Chem Toxicol. https://doi.org/10.1080/01480545.2020.1722690
Wang X, Zhang J-B, He K-J, Wang F, Liu C-F (2021) Advances of zebrafish in neurodegenerative disease: from models to drug discovery. Front Pharmacol 12:1802
Johnston HJ, Verdon R, Gillies S, Brown DM, Fernandes TF, Henry TB et al (2018) Adoption of in vitro systems and zebrafish embryos as alternative models for reducing rodent use in assessments of immunological and oxidative stress responses to nanomaterials. Crit Rev Toxicol 48(3):252–271
Truong L, Tilton SC, Zaikova T, Richman E, Waters KM, Hutchison JE et al (2013) Surface functionalities of gold nanoparticles impact embryonic gene expression responses. Nanotoxicology 7(2):192–201
Krishnaraj C, Harper SL, Yun SI (2016) In Vivo toxicological assessment of biologically synthesized silver nanoparticles in adult Zebrafish (Danio rerio). J Hazard Mater 301:480–491
Cha C, Shin SR, Annabi N, Dokmeci MR, Khademhosseini A (2013) Carbon-based nanomaterials: multifunctional materials for biomedical engineering. ACS Nano 7(4):2891–2897
Kumar V, Toffoli G, Rizzolio F (2013) Fluorescent carbon nanoparticles in medicine for cancer therapy. ACS Med Chem Lett 4(11):1012–1013
da Rocha AM, Kist LW, Almeida EA, Silva DGH, Bonan CD, Altenhofen S et al (2019) Neurotoxicity in zebrafish exposed to carbon nanotubes: effects on neurotransmitters levels and antioxidant system. Comp Biochem Physiol Toxicol Pharmacol CBP 218:30–35
Wang ZG, Zhou R, Jiang D, Song JE, Xu Q, Si J et al (2015) Toxicity of graphene quantum dots in zebrafish embryo. Biomed Environ Sci BES 28(5):341–351
Kang Y-F, Li Y-H, Fang Y-W, Xu Y, Wei X-M, Yin X-B (2015) Carbon quantum dots for zebrafish fluorescence imaging. Sci Rep 5:11835
Partha R, Conyers JL (2009) Biomedical applications of functionalized fullerene-based nanomaterials. Int J Nanomed 4:261–275
Montellano A, Da Ros T, Bianco A, Prato M (2011) Fullerene C(6)(0) as a multifunctional system for drug and gene delivery. Nanoscale 3(10):4035–4041
Beuerle F, Witte P, Hartnagel U, Lebovitz R, Parng C, Hirsch A (2007) Cytoprotective activities of water-soluble fullerenes in zebrafish models. J Exp Nanosci 2(3):147–170
Marta DA, Rodio M, Bartelmess J, Sancataldo G, Brescia R, Cella Zanacchi F et al (2016) Biocompatibility and biodistribution of functionalized carbon nano-onions (f-CNOs) in a vertebrate model. Sci Rep 6:33923
Liu Z, Tabakman S, Welsher K, Dai H (2009) Carbon nanotubes in biology and medicine: in vitro and in vivo detection, imaging and drug delivery. Nano Res 2(2):85–120
He H, Pham-Huy LA, Dramou P, Xiao D, Zuo P, Pham-Huy C (2013) Carbon nanotubes: applications in pharmacy and medicine. BioMed Res Int 2013:578090
Wang R, Alicea NM, Lee M Jr, Deutsch D, Miadzvedskaya L, Braun E et al (2016) Toxicity assessment and bioaccumulation in zebrafish embryos exposed to carbon nanotubes suspended in Pluronic(R) F-108. Nanotoxicology 10(6):689–698
Cheng J, Cheng SH (2012) Influence of carbon nanotube length on toxicity to zebrafish embryos. Int J Nanomed 7:3731–3739
Filho Jde S, Matsubara EY, Franchi LP, Martins IP, Rivera LM, Rosolen JM et al (2014) Evaluation of carbon nanotubes network toxicity in zebrafish (Danio rerio) model. Environ Res 134:9–16
Maes HM, Stibany F, Giefers S, Daniels B, Deutschmann B, Baumgartner W et al (2014) Accumulation and distribution of multiwalled carbon nanotubes in zebrafish (Danio rerio). Environ Sci Technol 48(20):12256–12264
Baker SN, Baker GA (2010) Luminescent carbon nanodots: emergent nanolights. Angew Chem 49(38):6726–6744
Pan D, Zhang J, Li Z, Wu M (2010) Hydrothermal route for cutting graphene sheets into blue-luminescent graphene quantum dots. Adv Mater 22(6):734–738
Shen J, Zhu Y, Chen C, Yang X, Li C (2011) Facile preparation and upconversion luminescence of graphene quantum dots. Chem Commun 47(9):2580–2582
Zhou J, Booker C, Li R, Zhou X, Sham TK, Sun X et al (2007) An electrochemical avenue to blue luminescent nanocrystals from multiwalled carbon nanotubes (MWCNTs). J Am Chem Soc 129(4):744–745
Liu C, Zhang P, Zhai X, Tian F, Li W, Yang J et al (2012) Nano-carrier for gene delivery and bioimaging based on carbon dots with PEI-passivation enhanced fluorescence. Biomaterials 33(13):3604–3613
Wang Z, Xia J, Zhou C, Via B, Xia Y, Zhang F et al (2013) Synthesis of strongly green-photoluminescent graphene quantum dots for drug carrier. Colloids Surf B Biointerfaces 112:192–196
Wang X, Sun X, Lao J, He H, Cheng T, Wang M et al (2014) Multifunctional graphene quantum dots for simultaneous targeted cellular imaging and drug delivery. Colloids Surf B Biointerfaces 122:638–644
Kalaiarasi S, Arjun P, Nandhagopal S, Brijitta J, Iniyan AM, Vincent SGP et al (2016) Development of biocompatible nanogel for sustained drug release by overcoming the blood brain barrier in zebrafish model. J Appl Biomed 14(2):157–169
Sivaji K, Kannan RR (2019) Polysorbate 80 coated gold nanoparticle as a drug carrier for brain targeting in zebrafish model. J Clust Sci 30(4):897–906
Mochalin VN, Shenderova O, Ho D, Gogotsi Y (2011) The properties and applications of nanodiamonds. Nat Nanotechnol 7(1):11–23
Perevedentseva E, Lin YC, Jani M, Cheng CL (2013) Biomedical applications of nanodiamonds in imaging and therapy. Nanomedicine 8(12):2041–2060
Chang C-C, Zhang B, Li C-Y, Hsieh C-C, Duclos G, Treussart F, Chang H-C (2012) Exploring cytoplasmic dynamics in zebrafish yolk cells by single particle tracking of fluorescent nanodiamonds," Proc. SPIE 8272, Advances in Photonics of Quantum Computing, Memory, and Communication V, 827205.
Lin YC, Wu KT, Lin ZR, Perevedentseva E, Karmenyan A, Lin MD et al (2016) Nanodiamond for biolabelling and toxicity evaluation in the zebrafish embryo in vivo. J Biophoton 9(8):827–836
Saeedi M, Eslamifar M, Khezri K, Dizaj SM (2019) Applications of nanotechnology in drug delivery to the central nervous system. Biomed Pharmacother 111:666–675
Saraiva C, Praça C, Ferreira R, Santos T, Ferreira L, Bernardino L (2016) Nanoparticle-mediated brain drug delivery: overcoming blood–brain barrier to treat neurodegenerative diseases. J Controll Release 235:34–47
Zhang F (2017) Grand challenges for nanoscience and nanotechnology in energy and health. Front Chem 5:80
Lowry GV, Avellan A, Gilbertson LM (2019) Opportunities and challenges for nanotechnology in the agri-tech revolution. Nat Nanotechnol 14(6):517–522
Arms L, Smith DW, Flynn J, Palmer W, Martin A, Woldu A et al (2018) Advantages and limitations of current techniques for analyzing the biodistribution of nanoparticles. Front Pharmacol 9:802
Baran A (2016) Nanotechnology: legal and ethical issues. Eng Manag Prod Serv 8(1):47–54
Rong J, He Y, Tang J, Qiao R, Lin S (2021) “Fishing” nano–bio interactions at the key biological barriers. Nanoscale 13(12):5954–5964
Li X, Ji X, Wang R, Zhao J, Dang J, Gao Y et al (2020) Zebrafish behavioral phenomics employed for characterizing behavioral neurotoxicity caused by silica nanoparticles. Chemosphere 240:1237
Li X, Liu B, Li X-L, Li Y-X, Sun M-Z, Chen D-Y et al (2014) SiO2 nanoparticles change colour preference and cause Parkinson’s-like behaviour in zebrafish. Sci Rep 4(1):3810
Li X, Liu X, Li T, Li X, Feng D, Kuang X et al (2017) SiO2 nanoparticles cause depression and anxiety-like behavior in adult zebrafish. RSC Adv 7(5):2953–2963
Nellore J, Pauline C, Amarnath K (2013) Bacopa monnieri phytochemicals mediated synthesis of platinum nanoparticles and its neurorescue effect on 1-methyl 4-phenyl 1,2,3,6 tetrahydropyridine-induced experimental parkinsonism in zebrafish. J Neurodegener Dis 2013:972391
Fu L, Chung R, Shi B (2019) Upconversion nanoparticle-based strategy for crossing the blood–brain barrier to treat the central nervous system disease. Methods Mol Biol 2054:263–282
Hu Q, Guo F, Zhao F, Fu Z (2017) Effects of titanium dioxide nanoparticles exposure on parkinsonism in zebrafish larvae and PC12. Chemosphere 173:373–379
Javed I, Peng G, Xing Y, Yu T, Zhao M, Kakinen A et al (2019) Inhibition of amyloid beta toxicity in zebrafish with a chaperone-gold nanoparticle dual strategy. Nat Commun 10(1):3780
Acknowledgements
We acknowledge Sathyabama Institute of Science and Technology for their support.
Funding
The first author SS acknowledges the financial support from DBT, Govt of India through DBT-RA Program in Biotechnology and Life Sciences” The corresponding author RRK is acknowledging the Department of Biotechnology (DBT) (San.No: BT/PR6765/NNT/28/618/2012) Ministry of Science and Technology, Govt. of India.
Author information
Authors and Affiliations
Contributions
Both SS and RRK conceived the idea. SS wrote the review. RRK critically analysed the review. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saleem, S., Kannan, R.R. Zebrafish: A Promising Real-Time Model System for Nanotechnology-Mediated Neurospecific Drug Delivery. Nanoscale Res Lett 16, 135 (2021). https://doi.org/10.1186/s11671-021-03592-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11671-021-03592-1