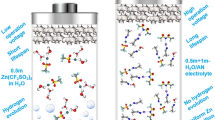
Abstract
Due to their outstanding power density, long cycle life and low cost, supercapacitors have gained much interest. As for supercapacitor electrodes, molybdenum nitrides show promising potential. Molybdenum nitrides, however, are mainly prepared as nanopowders via a chemical route and require binders for the manufacture of electrodes. Such electrodes can impair the performance of supercapacitors. Herein, binder-free chromium (Cr)-doped molybdenum nitride (Mo2N) TFEs having different Cr concentrations are prepared via a reactive co-sputtering technique. The Cr-doped Mo2N films prepared have a cubic phase structure of γ-Mo2N with a minor shift in the (111) plane. While un-doped Mo2N films exhibit a spherical morphology, Cr-doped Mo2N films demonstrate a clear pyramid-like surface morphology. The developed Cr-doped Mo2N films contain 0–7.9 at.% of Cr in Mo2N lattice. A supercapacitor using a Cr-doped Mo2N electrode having the highest concentration of Cr reveals maximum areal capacity of 2780 mC/cm2, which is much higher than that of an un-doped Mo2N electrode (110 mC/cm2). Furthermore, the Cr-doped Mo2N electrode demonstrates excellent cycling stability, achieving ~ 94.6% capacity retention for about 2000 cycles. The reactive co-sputtering proves to be a suitable technique for fabrication of binder-free TFEs for high-performance energy storage device applications.
Graphical Abstract

Similar content being viewed by others
Introduction
Electrochemical capacitors (ECs) or supercapacitors (SCs) are in high demand for a variety of applications, e.g., portable, wearable/flexible devices and electronic industries, as well as electric vehicles (EVs) due to their high-power density, fast charge–discharge and longer cycle life, [1,2,3,4]. Because of their enormous surface area, carbon-based materials (EDLC) including activated carbon remain the most used electrode material for ECs. However, carbon-based materials exhibit inferior energy density [5, 6]. Owing to their high capacitance, metal oxides/nitrides (pseudocapacitors) have been explored as alternative electrode materials for batteries and supercapacitors [5,6,7,8]. Yet, metal oxides reveal low electrical conductivity, which restricts their capacitance/capacity. There has been an urgent need, therefore, to replace carbon and metal oxide-based electrode materials with high-performance electrode materials [9].
Recently, sulfides and nitride-based materials have shown promise for the development of high-performance electrochemical ECs due to their outstanding electrochemical properties [10,11,12]. Because of their structural stability, unique physiochemical features, good electrocatalytic activities and superior electrical conductivity, metal nitrides are highly considered electrode material for ECs [12, 13]. So far, nitride-based electrodes, such as TiN [14, 15], VN [16,17,18,19], RuN [20], Mo2N [21,22,23], Ni2Mo3N [24], Ni3N [25], MnN@rGO [26] and CrN [27,28,29] electrodes, have been investigated for application in ECs. Molybdenum nitrides have been pursued as a viable electrode material for ECs owing to their great catalytic activity, excellent electrochemical behavior, low compressibility and high melting point. However, molybdenum nitrides as well as other metal nitrides are mainly prepared using chemical synthesis techniques, which result in mechanical instability and significant energy consumption. Moreover, studies of their electrochemical properties are still inadequate [30,31,32]. Nanomaterials prepared by chemical synthesis techniques in the form of nanopowder are in need of binders such as polyvinylidene fluoride (PVDF), polyvinyl alcohol (PVA) and carboxymethyl cellulose (CMC) to fabricate electrodes. Yet, such binders are known to hinder the performance of electrodes. Therefore, researchers are searching for other synthesis methods without using any binder [10, 25].
Due to the uniformity plus controlled stoichiometry of their coatings, good adhesion and well-defined structure, binder-free nitride-based thin film electrodes (TFEs) prepared by a sputtering technique are noted for producing high-performance, stable and flexible ECs [30]. Of note, the [111] grown molybdenum nitride films grown by using reactive direct current (DC) magnetron sputtering on titanium substrate at 400 °C demonstrate high areal capacitance of 55 mF cm−2 and excellent cycling stability of 100% capacitance retention after 2000 cycles [33]. Adalati et al. [34] synthesized a molybdenum and vanadium nitride binder-free thin film on a stainless steel substrate through reactive sputtering technique by varying the sputtering parameters such as Ar/N2 gas flow, applied DC power and deposition pressure. Vanadium nitride and molybdenum nitride were used to develop an asymmetrical device. These electrodes showed areal capacitances of 82.35 mF cm−2 (MoN) and 67.50 mF cm−2 (VN), respectively, with a capacitance retention of approximately 95.23%. Shi et al. [35] synthesized intercolumnar porous CrN TFEs at a substrate temperature of 250 °C under various N2 ratios, which displayed an areal capacitance of 41.7 mF cm−2 in H2SO4 electrolyte. Gao et al. [36] reported the synthesis of nanoporous CrN containing different ratios of metallic nickel (Ni), viz. 0, 30.4, 54.2 and 77.6 at.% (CrN-Ni) using arc ion plating. The nanoporous coating obtained using 54.2 at.% Ni, containing the CrN-Ni film, exhibited the highest capacitance of 58.5 mF cm−2 at 1.0 mA cm−2 greater than all other coatings, i.e., much higher than that of the as-deposited CrN electrode. In addition, this binder-free electrode provided an excellent capacitance retention rate. In general, doping of selective metal ions into host materials has proved to be a very good way to increase electrical conductivity and capacitance. However, there is still a gap in the development of binder-free metal nitride-based electrodes. To the best of our knowledge, no report has been published previously on the synthesis of binder-free Cr-doped Mo2N TFEs (TFEs) material for ECs. Thus, for the first time, novel binder-free Cr-substituted Mo2N TFEs have been developed for high-efficiency energy storage devices; their improved electrochemical performances are compared with existing metal nitrides-based electrodes.
Cr was chosen as a dopant in this work mainly because it is a metal having good electrical conductivity (0.0774 106/cm Ω and 7.9 × 106 S/m) and low ionic radii (0.62 Å). Cr is low in cost compared with other high conductive metals (Ag, Pt, V, Ru Ti and Ni). Besides, Cr is abundant (83% natural abundance) as well as corrosion-resistant. The addition of Cr in Mo2N synergistically alters the electronic states and creates better attainable active sites, enhancing the electrochemical performance and improving conductivity.
In the present work, nanopyramidal Cr-doped Mo2N (Cr/Mo2N) binder-free TFEs with different Cr doping concentrations: 0 to ~ 8 at.%, have been successfully synthesized via a reactive magnetron co-sputtering method for high-performance energy storage devices. Binder-free Mo2N TFEs show promise as active anode material for ECs. The impact of Cr on Mo2N as well as the microstructural and electrochemical charge storage properties of binder-free TFEs is discussed.
Experimental Section
Cr-Doped Mo2N Thin Films Deposition
Cr-doped MoN thin films are synthesized via a reactive co-sputtering technique (MP 300 sputter system, Plassys, France) along with 2 inch Cr and Mo targets having a purity of ~ 99.99% using argon (Ar+) as sputtering and nitrogen (N2) as reactive gases. In advance of deposition, the substrates: glass, silicon (100) and stainless steel 304, were cleaned (ultrasonically) by a standard cleaning process using acetone/ethanal and deionized water to eliminate the native oxide layers or any other impurities on the surface of the substrates. Subsequently, the substrates were dried and loaded into a sputtering chamber, as shown in Fig. 1.
After loading the substrates into the sputtering chamber, a base vacuum of 4 × 10−6 mbar was achieved using a turbo-molecular pump. Initially, before conducting deposition, both metallic targets were pre-sputtered in the Ar+ environment for ~ 10 min to eliminate residual native oxides over the target surfaces. During the growth of the film, a negative charge is applied to the target material (Mo and Cr) to initiate sputtering, which ionizes the working gas of the Ar+ and N sources. Positively charged Ar ions, generated in the plasma region, are rapidly attracted to the negatively biased Mo and Cr targets. Consequently, the atomic-sized Mo and Cr particles are ejected from the targets (Mo and Cr) as a result of the collision's momentum transfer. The plasmas negatively charged N2 atoms react with the Cr and Mo atoms; the resultant Cr/MoN thin film is ultimately deposited on the surface of the substrates. Herein, the Cr atoms introduced bind to the Mo2N crystal lattice directly through a three-body collision of Cr, Mo and N2 (Additional file 1: Fig. S1). All the films were prepared at a substrate temperature of 573 K (± 5) and working pressure of 9.8 mTorr. The Cr-doped Mo2N thin films were synthesized by fixing the Mo sputter power and varying Cr target power having a constant Ar/N2 flow rate. For the deposition of the pure Mo2N sample preparation, Mo target power remained unchanged. In Table 1, the detailed deposition parameters of this work are given. The un-doped Mo2N denotes the Mo2N film having 0 at.% of Cr. In addition, Cr/Mo2N-1, Cr/Mo2N-2, Cr/Mo2N-3 and Cr/Mo2N-4 denote the Cr-doped Mo2N films containing 3.35, 4.87, 6.21 and 7.90 at.% Cr, respectively.
Characterization Techniques
The sputter-deposited Cr-doped Mo2N thin films were investigated using various characterization techniques. Surface morphology and elemental composition of the Cr-doped Mo2N films were examined by field emission scanning electron microscope (FE-SEM, Carl Zeiss, Supra 55, Germany) and equipped with energy-dispersive X-ray spectrometer (EDS, Oxford instrumental), respectively. The crystallographic orientation and phase purity of the Cr-doped Mo2N films were characterized using grazing incidence X-ray diffractometer (XRD, D8 Advance, Bruker, Germany). XRD patterns were recorded at the diffraction angle of 2θ = 20°–70° using the Cu-Kα radiation wavelength (λ = 1.54 Å). To understand the oxidation states and chemical/electronic configuration of the thin films, X-ray photoelectron spectroscopy (XPS) technique using a ULVAC-PHI, Inc. (PHI Quantera SXM, USA) with an Al Kα X-ray source was adopted.
Electrochemical Measurements
Electrochemical behavior of the Cr-doped Mo2N TFEs was assessed using an electrochemical workstation (Bio-logic, SP-300, France) in a 3-electrode cell configuration in 1 M KOH electrolyte. The as-deposited Mo2N and Cr-doped Mo2N films on the stainless steel substrates were directly utilized as working electrodes. Ag/AgCl and platinum wire were used as reference and counter electrodes, respectively. Both cyclic voltammetry (CV) and galvanostatic charge–discharge (GCD) techniques were applied to calculate the areal capacity/capacitance with respect to different scan rates and current densities as well as the stability performance of the TFEs. The electrochemical impedance spectroscopy (EIS) technique was employed to examine the charge transfer mechanism of the film electrodes during electrochemical analysis.
Results and Discussion
Structural Characterization
Phase formation and crystal structure of the Mo2N and Cr-doped Mo2N thin films with thicknesses ranging from ~ 900 to 1400 nm (Additional file 1: Fig. S2) were initially investigated by XRD analysis. During XRD analysis, scan limit was fixed in the 2θ range of 20°–80°. In Fig. 2, the XRD patterns of as-deposited Mo2N and Cr-doped Mo2N thin films are displayed.
In both the un-doped and Cr-doped Mo2N thin films, three major diffraction peaks are noticed. Around 37.1°, a strong diffraction peak appeared and two low intensity peaks are observed around 43.1° and 79.2°, indexed to (111), (200) and (222) crystallographic orientations of Mo2N. All diffraction peaks were found to be well matched with γ-Mo2N (JCPDS file: PDF # 25-1366) having a cubic crystal system. The intensity of the diffraction peak 37.1° shifted slightly with respect to the higher amount of Cr doping concentration in the Mo2N film (Fig. 2b), confirming Cr ions in the Mo2N lattice and a sharp intensity peak at 37.1°, indicating high crystallinity of the prepared thin film samples. In Fig. 2b, the diffraction pattern of Cr-doped Mo2N, at 2θ around 37.1°, shifted to the right compared to the pristine Mo2N. Such a shift in the peak confirms the substitution of Cr in the Mo site with the presence of tensile stress in the as-deposited films [37, 38]. The expanding grain size caused tensile stress in the as-developed films, demonstrating a decreased lattice parameter (4.13 Å) relative to bulk Mo2N. In the as-grown films, the XRD pattern exhibited no peaks corresponding to the metallic Mo or Cr, or any other types of Mo/Cr nitrides. By substituting some of the Mo atoms with Cr, it is noted that Cr is completely in a solid solution with Mo2N and remains as a cubic structure for the entire Cr dopant concentration in the current investigation. Furthermore, the as-deposited thin films are a pure form of Mo2N, whereas the Cr-doped Mo2N films are highly crystalline in nature. No secondary phases were detected in the XRD analyses.
Surface Chemistry Studies
XPS was applied to the pristine Mo2N and Cr/Mo2N-4 film samples. The energy-dispersive X-ray spectroscopy technique was used to analyze the bulk composition of coatings. Moreover, the XPS technique can analyze surface chemical states at a depth as far as ~ 5 nm. Figure 3a, b shows the full scan survey spectra of the un-doped Mo2N and Cr/Mo2N-4 thin films. In Fig. 3c–g, the high-resolution spectra of all the elements present in the thin film samples are displayed. To avoid denitrification of Mo2N films due to Ar+ bombardment, no Ar+ ion etching was done before collecting the X-ray generated electrons. As illustrated in Fig. 3c, the Mo 3d core-level spectrum can be deconvoluted into four peaks, corresponding to Mo0 (~ 229 eV), Mo3+ (~ 230.1 eV) and Mo6+ (232.6 and 235.4 eV) species for the Cr–Mo–N film sample. As shown in Fig. 3f, the impure surface of the adventitious carbon (C 1s) at binding energy (BE) of 284.8 eV is denoted [39]. In Fig. 3g, the presence of oxide shows that the surface of the Mo species has been oxidized, indicating both lattice oxygen (Oa-530.5 eV) and surface observed oxygen (Ob-532.4 eV), which is mainly due to the contamination by surface oxygen upon exposure to air [40]. Further, two peaks at around 575.7 eV (Cr 2p3/2) and 586.4 eV (Cr 2p1/2) are revealed in the Cr-doped Mo2N thin film samples [41] and ascribed to Mo–Cr, demonstrating that Cr has been incorporated into the Mo2N host lattice.
In the XPS spectra, tiny peaks at 415 eV (Mo2N sample, Fig. 3a) and 413.7 eV (Cr/Mo2N-4 sample, Fig. 3b) are found. Such peaks are associated with the Mo 3p3/2 region. As shown in Fig. 3a, b, the positions of Mo 3d peaks for Mo–N and Mo–O bonds have shifted somewhat toward the low binding energy area. This behavior can be due to Cr doping, which can lower their bonding energy by sharing the Mo binding connection. In addition, one more primary peak can be detected for both Mo–N and Cr–Mo–N in the N 1s XPS spectra; the Mo–N bond is responsible for the peak at 397.4 eV (Fig. 3d). The N 1s peak for Cr–Mo–N has shifted to 396.8 eV (397.4 eV for the bare Mo–N), implying that the Cr element has been doped into the Mo2N lattice by replacing one of the Mo/N elements. In Fig. 3e, the Cr 2p XPS spectrum of Cr–Mo–N is seen to have a major peak at 575.7 eV. This peak differs significantly from the 574.2 eV of the Cr metal and the 576.0 eV of Cr, suggesting that the Cr dopants in the Mo–N lattice are Cr ions. In addition, the atomic percentages of the elements present in the thin film samples were roughly calculated from the XPS spectra as follows: Mo-33.18%, N-27.36%, C-15.72%, Cr-9.48% and O-14.26% for the Cr-doped Mo2N sample and Mo-39.96%, N-33.07%, O-12.39% and C-14.58% for Mo2N, respectively.
Morphological Studies
Morphological transformation of the pristine Mo2N and Cr-doped Mo2N thin films produced by the reactive co-sputtering process was evaluated using FE-SEM analysis. In Fig. 4a–e, FE-SEM images of the un-doped Mo2N and Cr-doped Mo2N thin films samples prepared at different concentrations of Cr doping are displayed, respectively. In Fig. 4a, the pure Mo2N thin film sample exhibits an agglomerated granular microstructure having a smooth surface; average particles are 20–30 nm in size. When doping concentration increases, all Cr/Mo2N thin film samples signify that the particles are densely packed together and are spread uniformly over the surface (Fig. 4b–e). The FE-SEM images of the Cr-doped Mo2N samples reveal a triangular pyramidal like surface morphology [42, 43].
Elemental Composition Studies
To examine both the elemental distribution and formation of Mo2N and Cr-doped Mo2N thin films, FE-SEM and EDS analyses were carried out. In Fig. 5, EDS reports of the as-prepared thin film samples are depicted. In Fig. 5a, the EDS spectra, viz. the Mo and N signals, can be seen for the un-doped Mo2N. In Fig. 5b–e, Cr, Mo and N signals are found in the Cr-doped Mo2N thin films. As Cr doping concentration increased, the intensity of the Cr peak increased, confirming the successful formation of the Cr-doped Mo2N thin films. In Fig. 5, the atomic percentiles for the Cr-doped Mo2N samples are shown. Besides, the EDS elemental color mapping images of Mo2N and Cr-doped Mo2N TFEs are highlighted, which verify the uniform deposition and distribution of Mo and N elements in the Mo2N thin film sample. Mo, Cr and N elements are all present in the Cr-doped Mo2N thin film samples (Additional file 1: Fig. S3).
Electrochemical Supercapacitor Performances
Electrochemical performance of the pristine Mo2N and Cr-doped Mo2N binder-free TFEs was carried out in 1 M KOH aqueous electrolyte under room temperature in a three-electrode cell setup. The applied voltage window of − 1.2 to − 0.2 V was fixed for both CV and GCD analysis.
Cyclic Voltammetry Analysis
The as-deposited Mo2N and Cr-doped Mo2N TFEs were further characterized via CV analysis under different scan rates (20–80 mV/s). Initially, both the stability and reversibility of the electrodes were examined, applying 10 CV cycles at a fixed scan rate of 50 mV/s. In Fig. 6, the CV curves of the bare stainless steel substrate, as-deposited pristine Mo2N and Cr-doped Mo2N TFEs are displayed. The shape of the CV curves for all the Cr-doped Mo2N TFEs is almost similar, and the higher doping concentration of Cr/Mo2N-4 electrode exhibits the bigger CV area compared to pristine Mo2N indicating the excellent capacity behavior, outstanding reversibility and rate capability [34]. It is also noted that the CV curves of the metal nitride-based electrodes revealed a quasi-rectangular shape having a superior active surface area even at lower scan rates, suggesting excellent charge storage behavior and high-rate capability [44,45,46,47]. The areal capacity of the Mo2N and Cr-doped Mo2N-based TFEs can be calculated from the CV curves:
where Qa is the areal capacity (mC/cm2), I is the current (A), A is the exposed active area of the electrode (cm2), and ν is the scan rate (mV s−1). Thus, via CV analysis, the maximum areal capacities of Cr/Mo2N-4 are found to be 2780 mC/cm2, Cr-doped Mo2N-3: 2220 mC/cm2, Cr-doped Mo2N-2: 1233 mC/cm2, Cr-doped Mo2N-1:960 mC/cm2 at the scan rate of 20 mV/s. The measured areal capacities of the battery-type Cr-doped Mo2N electrodes are remarkably greater than those of the un-doped Mo2N thin film electrode (110 mC/cm2), demonstrating the superior charge storage performance of the binder-free electrodes made of other metal nitrides [48, 49]. The increased areal capacity of the grown Cr-doped Mo2N TFEs may well be caused by the electrolyte ions' increased mobility at the interface between the aqueous electrolyte and active electrode, as well as the synergetic contribution of both Cr and Mo2N. In Fig. 7a, it is noted that the areal capacity values were found to be substantially greater than those of other metal nitrides-based electrodes (VN, CrN, TiN, etc.) that had previously been published. In Table 2, the performance of the electrodes are summarized.
a Areal capacities for the Cr-doped Mo2N tested under various scan rates, b plot of diffusion and capacitive contribution of Cr/Mo2N-4 electrode evaluated, at a scan rate of 80 mV/s, and c illustrating the diffusive and capacitive contributions of different doping concentration of Cr-doped Mo2N electrodes, at a fixed scan rate of 80 mV/s
Moreover, to better understand the charge storage behavior of Cr-doped Mo2N TFEs via CV analysis, Dunn’s approach is adopted to recognize the two different charge storage contributions: (i) surface-controlled and (ii) diffusion-controlled, which states that the total current at a given potential is the sum of the diffusive and capacitive currents investigated [60]:
where k1 and k2 are differentiated as surface- and diffusion-controlled contributions of the developed TFEs, respectively, i(V) is the current of the given potential, ν is the applied scan rate, and k1 and k2 values are calculated by plotting the graph between and i(V)/ν0.5 and (ν)0.5. In Fig. 7b, the CV curves of the diffusive and capacitive contribution of the Cr-doped Mo2N-4 electrode evaluated at a scan rate of 80 mV/s are shown, revealing ~ 83% diffusive contribution to accumulate the charge of the electrode. Besides, the diffusive contribution of Cr/Mo2N-1, Cr/Mo2N-2, Cr/Mo2N-3 and Cr/Mo2N-4 TFEs is shown to be ~ 83%, ~ 66%, ~ 61% and ~ 59%, respectively (Fig. 7c). These findings suggest that the as-developed nitride-based electrodes have excellent charge storage behavior, with almost mixed contributions (capacitive and diffusive) to total charge storage for lower doping concentrations of Cr in Mo2N electrodes and the maximum diffusive contribution behavior observed for the higher doping percent of Cr in Mo2N. As a result, the Cr-doped Mo2N electrodes are considered to be potential candidates for high-performance energy storage device applications due to the synergetic contribution between Cr dopant and Mo2N. Additionally, the mechanism of the electrochemical reaction between the active electrode material and the electrolyte can be described as follows:
Galvanostatic Charge–Discharge Analysis
The charge–discharge (CD) performance of the as-prepared electrodes was further examined via GCD studies in 1 M KOH aqueous electrolyte. In Fig. 8a–e, the GCD profiles of Mo2N and Cr-doped Mo2N TFEs conducted at different current densities ranging from 1 to 3 mA/cm2 in a fixed voltage window of − 1.2 to − 0.2 V are presented. In the GCD analysis, the nonlinear shape of the charge–discharge profiles is clearly visible, indicating an ideal capacitive nature; a similar trend of charge–discharge patterns has been seen in previous studies [45, 61]. Hence, the energy storage in the Cr-doped Mo2N TFEs is attributed to both physisorption of the electric double layer (EDL) and faradaic reaction. The Cr-doped Mo2N electrode shows a dramatically longer discharging time compared to the pristine Mo2N electrode, which may account for the substitution of Cr in the Mo2N lattice. The areal capacity of the TFEs, according to the GCD analysis, is obtained for various current densities and can be expressed as:
where Qa is the areal capacity (mC/cm2), I is the current (A), Δt is the time difference between the charge/discharge profile (s), and A is the exposure active area of the electrode (cm2).
Based on the GCD results, the maximum areal capacity of 243 mC/cm2 for the Cr-doped Mo2N and 93 mC/cm2 for the un-doped Mo2N was attained in 1 mA/cm2 current density. All the Cr-doped Mo2N TFEs demonstrated high areal capacities more than the un-doped Mo2N TFEs (Fig. 8f). These results proved to be much higher than previously reported nitride-based electrodes: CrN [36], Mn3N2 [57], Co3N [62], Nb4N5 [63], TiN [64], HfN [65], GaN [66], VN [67] and W2N [68]. As doping concentration of Cr increased, areal capacity values increased, with respect to various current densities. In Fig. 9f, it is clear that when current density increased, areal capacities decreased, due to insufficient time for complete ion exchange in the electrolyte/electrode interface at higher current densities [35]. CD profiles show a curvy and symmetrical linear shape and indicate the superior charge storage behavior of the electrodes having low ohmic potential loss (IR drop), reflecting the great capacity and reversibility of the electrodes [55, 69]. Such positive CD characteristics arose owing to the synergistic effect of doping Cr with the Mo2N electrode. The high-efficiency behavior of the as-prepared Cr-doped Mo2N thin film-based electrodes verifies their potential for use as high-performance ECs.
Cycling Stability and Impedance Analysis
Cycling behavior and rate performance are essential parameters for the practical application of energy storage devices. In Fig. 9a, the Cr/Mo2-4 electrode measured up to 2000 CV cycles, demonstrating maximum capacity retention of ~ 94.6%. As the number of cycles increased (up to 2000), the rate capability of the electrodes is seen to decrease slowly, which indicates that the prepared nitride-based electrodes are long-lasting even at higher cycles: significantly higher than previously studied metal nitrides such as Nb4N5 [46], VN [70] and TiN [71]. It is acknowledged that the improved electrochemical performance of the Cr-doped Mo2N electrodes can be attributed to the synergistic effect between the dopant and host materials.
Finally, to understand electrochemical kinetics such as the charge transfer process between as-prepared nitride-based electrodes and electrolyte interface, EIS studies were carried out. In Fig. 9b, it is found that the Nyquist plot of Mo2N and Cr-doped Mo2N TFEs performed at a frequency range of 1 Hz–100 MHz with an amplitude of 10 mV. According to the electrochemical Nyquist plot, the curve can be separated into three components: electrolyte resistance, a vertical line in the low-frequency zone and a semicircle in the high-frequency zone. The electrolyte resistance, namely the ionic resistance of the electrolyte, intrinsic resistance of the electrode and interface resistance, is represented by the intersection with the x-coordinate in the high-frequency region [72]. The vertical/inclined line in the low-frequency zone of as-prepared nitride-based electrodes is due to the diffusion of ions at the electrode–electrolyte interface, indicating the remarkable conductive behavior of the electrode [34]. The semicircle in the high-frequency zone represents charge transfer resistance (Rct) in the electrode and electrolyte interface along with the Rct of 521 Ω for the un-doped Mo2N sample and 130 Ω for the Cr-doped Mo2N thin film sample. During the electrochemical process, the decrease in charge transfer resistance after doping Mo2N with Cr may be due to rapid electron and ion transfer and excellent electrolyte accessibility. Such an outcome demonstrates that the Cr-doped Mo2N TFEs can increase the electrode reaction kinetics of binder-free ECs.
Conclusions
In summary, the nanopyramidal-shaped Cr-doped Mo2N binder-free TFEs were prepared via a reactive co-sputtering technique; their microstructural and electrochemical energy storage properties were systematically elucidated. It is significant that the doping effect of the Cr transition metal played an important role in enhancing the electrochemical energy storage performance of the Mo2N TFEs. The obtained un-doped and Cr-doped Mo2N thin films were investigated in detail via XRD, XPS, FE-SEM with EDS analyses. CV studies demonstrated that the ~ 7.9 at.% Cr-doped Mo2N TFE exhibited a maximum areal capacity of 2780 mC/cm2, which proved to be much greater than the pristine Mo2N electrode and other nitride-based TFEs shown in prior investigations. Furthermore, the GCD study demonstrated that the charge–discharge profiles have a symmetrical linear shape, exhibiting outstanding discharge behavior, with a areal capacity of 243 mC/cm2. The higher doping concentration of Cr-doped Mo2N electrode displayed outstanding cycling stability, with a capacity loss of only 5.4% after 2000 CV cycles. The nitride-based TFEs prepared by the reactive co-sputtering technique were found to be a simple technique for developing high-performance binder-free electrodes that proved to have excellent cycling stability, displaying superior electrochemical characteristics for highly valued future energy storage devices.
Availability of Data and Materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Kang L, Zhang M, Zhang J, Liu S, Zhang N, Yao W, Ye Y, Luo C, Gong Z, Wang C, Zhou X (2020) Dual-defect surface engineering of bimetallic sulfide nanotubes towards flexible asymmetric solid-state supercapacitors. J Mater Chem A 8(45):24053–24064. https://doi.org/10.1039/D0TA08979F
Liu S, Kang L, Zhang J, Jung E, Lee S, Jun SC (2020) Structural engineering and surface modification of MOF-derived cobalt-based hybrid nanosheets for flexible solid-state supercapacitors. Energy Storage Mater 1(32):167–177. https://doi.org/10.1016/j.ensm.2020.07.017
Liu S, Kang L, Hu J, Jung E, Henzie J, Alowasheeir A, Zhang J, Miao L, Yamauchi Y, Jun SC (2022) Realizing superior redox kinetics of hollow bimetallic bulfide nanoarchitectures by defect-induced manipulation toward flexible solid-state supercapacitors. Small 18(5):2104507. https://doi.org/10.1002/smll.202104507
Sahoo S, Ratha S, Rout CS, Nayak SK (2022) Self-charging supercapacitors for smart electronic devices: a concise review on the recent trends and future sustainability. J Mater Sci 57:1–42. https://doi.org/10.1007/s10853-022-06875-9
Patra A, Namsheer K, Jose JR, Sahoo S, Chakraborty B, Rout CS (2021) Understanding the charge storage mechanism of supercapacitors: in situ/operando spectroscopic approaches and theoretical investigations. J Mater Chem A 9(46):25852–25891. https://doi.org/10.1039/D1TA07401F
Wang G, Zhang L, Zhang J (2012) A review of electrode materials for electrochemical supercapacitors. Chem Soc Rev 41(2):797–828. https://doi.org/10.1039/C1CS15060J
Liu S, Kang L, Hu J, Jung E, Zhang J, Jun SC, Yamauchi Y (2021) Unlocking the potential of oxygen-deficient copper-doped Co3O4 nanocrystals confined in carbon as an advanced electrode for flexible solid-state supercapacitors. ACS Energy Lett 6(9):3011–3019. https://doi.org/10.1021/acsenergylett.1c01373
Kandasamy M, Sahoo S, Nayak SK, Chakraborty B, Rout CS (2021) Recent advances in engineered metal oxide nanostructures for supercapacitor applications: experimental and theoretical aspects. J Mater Chem A 9(33):17643–17700. https://doi.org/10.1039/D1TA03857E
Pramitha A, Raviprakash Y (2022) Recent developments and viable approaches for high-performance supercapacitors using transition metal-based electrode materials. J Energy Storage 49:104120. https://doi.org/10.1016/j.est.2022.104120
Sahoo S, Krishnamoorthy K, Pazhamalai P, Kim SJ (2018) Copper molybdenum sulfide anchored nickel foam: a high performance, binder-free, negative electrode for supercapacitors. Nanoscale 10(29):13883–13888. https://doi.org/10.1039/C8NR03998D
Sahoo S, Naik KK, Late DJ, Rout CS (2017) Electrochemical synthesis of a ternary transition metal sulfide nanosheets on nickel foam and energy storage application. J Alloys Compd 695:154–161. https://doi.org/10.1016/j.jallcom.2016.10.163
Zhou Y, Guo W, Li T (2019) A review on transition metal nitrides as electrode materials for supercapacitors. Ceram Int 45(17):21062–21076. https://doi.org/10.1016/j.ceramint.2019.07.151
Pallavolu MR, Anil Kumar Y, Nallapureddy RR, Goli HR, Narayan Banerjee A, Joo SW (2022) In situ design of porous vanadium nitride@carbon nanobelts: a promising material for high-performance asymmetric supercapacitors. Appl Surf Sci 575:151734. https://doi.org/10.1016/j.apsusc.2021.151734
Achour A, Ducros JB, Porto RL, Boujtita M, Gautron E, Le Brizoual L et al (2014) Hierarchical nanocomposite electrodes based on titanium nitride and carbon nanotubes for micro-supercapacitors. Nano Energy 7:104–113. https://doi.org/10.1016/j.nanoen.2014.04.008
Achour A, Porto RL, Soussou M-A, Islam M, Boujtita M, Aissa KA et al (2015) Titanium nitride films for micro-supercapacitors: effect of surface chemistry and film morphology on the capacitance. J Power Sources 300:525–532. https://doi.org/10.1016/j.jpowsour.2015.09.012
Yan Y, Li B, Guo W, Pang H, Xue H (2016) Vanadium based materials as electrode materials for high performance supercapacitors. J Power Sources 329:148–169. https://doi.org/10.1016/j.jpowsour.2016.08.039
Glushenkov AM, Hulicova-Jurcakova D, Llewellyn D, Lu GQ, Chen Y (2010) Structure and capacitive properties of porous nanocrystalline VN prepared by temperature-programmed ammonia reduction of V2O5. Chem Mater 22(3):914–921. https://doi.org/10.1021/cm901729x
Bondarchuk O, Morel A, Bélanger D, Goikolea E, Brousse T, Mysyk R (2016) Thin films of pure vanadium nitride: evidence for anomalous non-faradaic capacitance. J Power Sources 324:439–446. https://doi.org/10.1016/j.jpowsour.2016.05.093
Morel A, Borjon-Piron Y, Porto RL, Brousse T, Bélanger D (2016) Suitable conditions for the use of vanadium nitride as an electrode for electrochemical capacitor. J Electrochem Soc 163(6):A1077–A1082. https://doi.org/10.1149/2.1221606jes
Bouhtiyya S, Lucio Porto R, Laïk B, Boulet P, Capon F, Pereira-Ramos JP et al (2013) Application of sputtered ruthenium nitride thin films as electrode material for energy-storage devices. Scr Mater 68(9):659–662. https://doi.org/10.1016/j.scriptamat.2013.01.030
Dubal DP, Abdel-Azeim S, Chodankar NR, Han Y-K (2019) Molybdenum nitride nanocrystals anchored on phosphorus-incorporated carbon fabric as a negative electrode for high-performance asymmetric pseudocapacitor. IScience 16:50–62. https://doi.org/10.1016/j.isci.2019.05.018
Shah SIU, Hector AL, Owen JR (2014) Redox supercapacitor performance of nanocrystalline molybdenum nitrides obtained by ammonolysis of chloride- and amide-derived precursors. J Power Sources 266:456–463. https://doi.org/10.1016/j.jpowsour.2014.05.045
Xiong Z, Yang J, Gao Z, Yang Q, Shi D (2020) Orthorhombic Mo3N2 nanobelts with improved electrochemical properties as electrode material for supercapacitors. Results Phys 16:102941. https://doi.org/10.1016/j.rinp.2020.102941
Kumar R, Bhuvana T, Sharma A (2020) Ammonolysis synthesis of nickel molybdenum nitride nanostructures for high-performance asymmetric supercapacitors. New J Chem 44(33):14067–14074. https://doi.org/10.1039/D0NJ01693D
Prasad S, Durai G, Devaraj D, AlSalhi MS, Theerthagiri J, Arunachalam P et al (2018) 3D nanorhombus nickel nitride as stable and cost-effective counter electrodes for dye-sensitized solar cells and supercapacitor applications. RSC Adv 8(16):8828–8835. https://doi.org/10.1039/C8RA00347E
Sridhar V, Park H (2019) Manganese nitride stabilized on reduced graphene oxide substrate for high performance sodium ion batteries, super-capacitors and EMI shielding. J Alloys Compd 808:151748. https://doi.org/10.1016/j.jallcom.2019.151748
Guerra A, Haye E, Achour A, Harnois M, Hadjersi T, Colomer JF, Pireaux JJ, Lucas S, Boukherroub R (2019) High performance of 3D silicon nanowires array@CrN for electrochemical capacitors. Nanotechnology 31(3):035407. https://doi.org/10.1088/1361-6528/ab4963
Wei B, Liang H, Zhang D, Wu Z, Qi Z, Wang Z (2017) CrN thin films prepared by reactive DC magnetron sputtering for symmetric supercapacitors. J Mater Chem A 5(6):2844–2851. https://doi.org/10.1039/C6TA09985H
Haye E, Achour A, Guerra A, Moulaï F, Hadjersi T, Boukherroub R, Panepinto A, Brousse T, Pireaux JJ, Lucas S (2019) Achieving on chip micro-supercapacitors based on CrN deposited by bipolar magnetron sputtering at glancing angle. Electrochim Acta 324:134890. https://doi.org/10.1016/j.electacta.2019.134890
Zhong Y, Xia X, Shi F, Zhan J, Tu J, Fan HJ (2016) Transition metal carbides and nitrides in energy storage and conversion. Adv Sci 3(5):1500286. https://doi.org/10.1002/advs.201500286
Kang Y, Deng C, Chen Y, Liu X, Liang Z, Li T, Hu Q, Zhao Y (2020) Binder-free electrodes and their application for Li-ion batteries. Nanoscale Res Lett 15(1):1–9. https://doi.org/10.1186/s11671-020-03325-w
Gerard O, Numan A, Krishnan S, Khalid M, Subramaniam R, Kasi R (2022) A review on the recent advances in binder-free electrodes for electrochemical energy storage application. J Energy Storage 50:104283. https://doi.org/10.1016/j.est.2022.104283
Chen L, Liu C, Zhang Z (2017) Novel [111] oriented γ-Mo2N thin films deposited by magnetron sputtering as an anode for aqueous micro-supercapacitors. Electrochim Acta 245:237–248. https://doi.org/10.1016/j.electacta.2017.05.102
Adalati R, Kumar A, Kumar Y, Chandra RA (2020) High-performing asymmetric supercapacitor of molybdenum nitride and vanadium nitride thin films as binder-free electrode grown through reactive sputtering. Energy Technol 8(10):2000466. https://doi.org/10.1002/ente.202000466.35
Shi J, Jiang B, Li C, Yan F, Wang D, Yang C, Wang X, Liu Z (2021) Sputtered chromium nitride films with finely tuned intra-and intercolumnar porosities as pseudocapacitive electrode for supercapacitors. Surf Coat Technol 405:126535. https://doi.org/10.1016/j.surfcoat.2020.126535
Gao Z, Wan Z, Wu Z, Huang X, Li H, Zhang TF et al (2021) Synthesis and electrochemical properties of nanoporous CrN thin film electrodes for supercapacitor applications. Mater Des 209:109949. https://doi.org/10.1016/j.matdes.2021.109949
ul Haq M, Zhang Z, Wen Z, Khan S, ud Din S, Rahman N et al (2019) Humidity sensor based on mesoporous Al-doped NiO ultralong nanowires with enhanced ethanol sensing performance. J Mater Sci Mater 30(7):7121–7134. https://doi.org/10.1007/s10854-019-01030-8
Irum S, Andleeb S, Sardar S, Mustafa Z, Ghaffar G, Mumtaz M et al (2021) Chemical synthesis and antipseudomonal activity of Al-doped NiO nanoparticles. Front Mater 8:673458. https://doi.org/10.3389/fmats.2021.673458
Greczynski G, Hultman L (2020) X-ray photoelectron spectroscopy: towards reliable binding energy referencing. Prog Mater Sci 107:100591. https://doi.org/10.1016/j.pmatsci.2019.100591
Huang Y, Ge J, Hu J, Zhang J, Hao J, Wei Y (2018) Nitrogen-doped porous molybdenum carbide and phosphide hybrids on a carbon matrix as highly effective electrocatalysts for the hydrogen evolution reaction. Adv Energy Mater 8(6):1701601. https://doi.org/10.1002/aenm.201701601
Zhao W, DiSalvo FJ (2015) Direct access to macroporous chromium nitride and chromium titanium nitride with inverse opal structure. Chem Commun 51(23):4876–4879. https://doi.org/10.1039/C4CC09564B
Kumar A, Sanger A, Kumar A, Chandra R (2017) Single-step growth of pyramidally textured NiO nanostructures with improved supercapacitive properties. Int J Hydrog Energy 42(9):6080–6087. https://doi.org/10.1016/j.ijhydene.2016.11.036
Qi Z, Wei B, Wang J, Yang Y, Wang Z (2019) Nanostructured porous CrN thin films by oblique angle magnetron sputtering for symmetric supercapacitors. J Alloys Compd 806:953–959. https://doi.org/10.1016/j.jallcom.2019.07.325
Su H, Xiong T, Tan Q, Yang F, Appadurai PBS, Afuwape AA et al (2020) Asymmetric pseudocapacitors based on interfacial engineering of vanadium nitride hybrids. Nanomaterials 10(6):1141. https://doi.org/10.3390/nano10061141
Hassan M, Gondal MA, Cevik E, Qahtan TF, Bozkurt A, Dastageer MA (2020) High performance pliable supercapacitor fabricated using activated carbon nanospheres intercalated into boron nitride nanoplates by pulsed laser ablation technique. Arab J Chem 13(8):6696–6707. https://doi.org/10.1016/j.arabjc.2020.06.024
Cui H, Zhu G, Liu X, Liu F, Xie Y, Yang C et al (2015) Niobium nitride Nb4N5 as a new high-performance electrode material for supercapacitors. Adv Sci 2(12):1500126. https://doi.org/10.1002/advs.201500126
Li X-S, Xu M-M, Yang Y, Huang Q-B, Wang X-Y, Ren J-L et al (2019) MnO2@Corncob carbon composite electrode and all-solid-state supercapacitor with improved electrochemical performance. Materials 12(15):2379. https://doi.org/10.3390/ma12152379
Nandi DK, Sahoo S, Kim TH, Cheon T, Sinha S, Rahul R, Jang Y, Bae JS, Heo J, Shim JJ, Kim SH (2018) Low temperature atomic layer deposited molybdenum nitride–Ni–foam composite: an electrode for efficient charge storage. Electrochem Commun 1(93):114–118. https://doi.org/10.1016/j.elecom.2018.07.003
Simon P, Gogotsi Y, Dunn B (2014) Where do batteries end and supercapacitors begin? Science 343(6176):1210–1211. https://doi.org/10.1126/science.1249625
Shinde PA, Chodankar NR, Abdelkareem MA, Han YK, Olabi AG (2022) Nitridation-induced in situ coupling of Ni-Co4N particles in nitrogen-doped carbon nanosheets for hybrid supercapacitors. Chem Eng J 428:131888. https://doi.org/10.1016/j.cej.2021.131888
Durai G, Kuppusami P, Theerthagiri J (2018) Microstructural and supercapacitive properties of reactive magnetron co-sputtered Mo3N2 electrodes: effects of Cu doping. Mater Lett 220:201–204. https://doi.org/10.1016/j.matlet.2018.02.120
Liu X, Zang W, Guan C, Zhang L, Qian Y, Elshahawy AM, Zhao D, Pennycook SJ, Wang J (2018) Ni-doped cobalt–cobalt nitride heterostructure arrays for high-power supercapacitors. ACS Energy Lett 3(10):2462–2469. https://doi.org/10.1021/acsenergylett.8b01393
Ouendi S, Robert K, Stiévenard D, Brousse T, Roussel P, Lethien C (2019) Sputtered tungsten nitride films as pseudocapacitive electrode for on chip micro-supercapacitors. Energy Storage Mater 20:243–252. https://doi.org/10.1016/j.ensm.2019.04.006
Xie Y, Tian F (2017) Capacitive performance of molybdenum nitride/titanium nitride nanotube array for supercapacitor. Mater Sci Eng B 215:64–70. https://doi.org/10.1016/j.mseb.2016.11.005
Achour A, Lucio-Porto R, Solaymani S, Islam M, Ahmad I, Brousse T (2018) Reactive sputtering of vanadium nitride thin films as pseudo-capacitor electrodes for high areal capacitance and cyclic stability. J Mater Sci Mater 29(15):13125–13131. https://doi.org/10.1007/s10854-018-9435-z
Ruan Y, Lv L, Li Z, Wang C, Jiang J (2017) Ni nanoparticles@Ni–Mo nitride nanorod arrays: a novel 3D-network hierarchical structure for high areal capacitance hybrid supercapacitors. Nanoscale 9(45):18032–18041. https://doi.org/10.1039/C7NR05560A
Durai G, Kuppusami P, Maiyalagan T, Ahila M, Vinoth Kumar P (2019) Supercapacitive properties of manganese nitride thin film electrodes prepared by reactive magnetron sputtering: effect of different electrolytes. Ceram Int 45(14):17120–17127. https://doi.org/10.1016/j.ceramint.2019.05.265
Shi J, Jiang B, Liu Z, Li C, Yan F, Liu X et al (2021) Effects of specific surface area of electrode and different electrolyte on capacitance properties in nano porous-structure CrN thin film electrode for supercapacitor. Ceram Int 47(13):18540–18549. https://doi.org/10.1016/j.ceramint.2021.03.177
Shi J, Jiang B, Li C, Liu Z, Yan F, Liu X et al (2022) Study on capacitance properties of the sputtered carbon doped titanium nitride electrode material for supercapacitor. Vacuum 198:110893. https://doi.org/10.1016/j.vacuum.2022.110893
Augustyn V, Simon P, Dunn B (2014) Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ Sci 7(5):1597–1614. https://doi.org/10.1039/C3EE44164D
Majumder M, Choudhary RB, Thakur AK, Karbhal I (2017) Impact of rare-earth metal oxide (Eu2O3) on the electrochemical properties of a polypyrrole/CuO polymeric composite for supercapacitor applications. RSC Adv 7:20037–20048. https://doi.org/10.1039/C7RA01438D
Zhang H, Hu W, Wei B, Zheng J, Qi Z, Wang Z (2021) Freestanding Co3N thin film for high performance supercapacitors. Ceram Int 47(3):3267–3271. https://doi.org/10.1016/j.ceramint.2020.09.166
Gao B, Xiao X, Su J, Zhang X, Peng X, Fu J et al (2016) Synthesis of mesoporous niobium nitride nanobelt arrays and their capacitive properties. Appl Surf Sci 383:57–63. https://doi.org/10.1016/j.apsusc.2016.04.173
Wang H-Y, Li B, Teng J-X, Zhu H-L, Qi Y-X, Yin L-W et al (2017) N-doped carbon-coated TiN exhibiting excellent electrochemical performance for supercapacitors. Electrochim Acta 257:56–63. https://doi.org/10.1016/j.electacta.2017.10.066
Gao Z, Wu Z, Zhao S, Zhang T, Wang Q (2019) Enhanced capacitive property of HfN film electrode by plasma etching for supercapacitors. Mater Lett 235:148–152. https://doi.org/10.1016/j.matlet.2018.10.032
Wang S, Zhang L, Sun C, Shao Y, Wu Y, Lv J et al (2016) Gallium nitride crystals: novel supercapacitor electrode materials. Adv Mater 28(19):3768–3776. https://doi.org/10.1002/adma.201600725
Durai G, Kuppusami P, Maiyalagan T, Theerthagiri J, Vinoth Kumar P, Kim H-S (2019) Influence of chromium content on microstructural and electrochemical supercapacitive properties of vanadium nitride thin films developed by reactive magnetron co-sputtering process. Ceram Int 45(10):12643–12653. https://doi.org/10.1016/j.ceramint.2019.02.170
Prakash R, Kumar A, Pandey A, Kaur D (2019) Binder free and high performance of sputtered tungsten nitride thin film electrode for supercapacitor device. Int J Hydrog Energy 44(21):10823–10832. https://doi.org/10.1016/j.ijhydene.2019.02.005
Lang X-Y, Liu B-T, Shi X-M, Li Y-Q, Wen Z, Jiang Q (2016) Ultrahigh-power pseudocapacitors based on ordered porous heterostructures of electron-correlated oxides. Adv Sci 3(5):1500319. https://doi.org/10.1002/advs.201500319
Wang Y, Jiang M, Yang Y, Ran F (2016) Hybrid electrode material of vanadium nitride and carbon fiber with cigarette butt/metal ions wastes as the precursor for supercapacitors. Electrochim Acta 222:1914–1921. https://doi.org/10.1016/j.electacta.2016.12.003
Choi D, Kumta PN (2006) Nanocrystalline TiN derived by a two-step halide approach for electrochemical capacitors. J Electrochem Soc 153(12):A2298. https://doi.org/10.1149/1.2359692
Ning W-W, Chen L-B, Wei W-F, Chen Y-J, Zhang X-Y (2020) NiCoO2/NiCoP@Ni nanowire arrays: tunable composition and unique structure design for high-performance winding asymmetric hybrid supercapacitors. Rare Met 39(9):1034–1044. https://doi.org/10.1007/s12598-020-01374-9
Acknowledgements
The Energy Storage Cluster, Chulalongkorn University, is acknowledged.
Funding
This work was supported by The Program Unit for Human Resources & Institutional Development, Research and Innovation (B16F640166, B05F640153); Chulalongkorn Academic Advancement into its 2nd Century Project for Postdoctoral Fellowship (C2F); Hokkaido University and Accelerating Social Implementation for SDGs Achievement (B) (aXis) from Japan Science and Technology Agency; and Grant-in-Aid for Scientific Research in Priority Area (21H00138 and 19H05162).
Author information
Authors and Affiliations
Contributions
DG contributed to conceptualization; investigation; data curation; formal analysis; writing—original draft; and writing—review and editing. NP, KKC, MTN, TY and JQ equally contributed to formal analysis and writing—review and editing. SK was involved in formal analysis; funding acquisition; supervision; writing—original draft; and writing—review and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Growth mechanism of Cr doped Mo2N TFEs through sputtering technique; FESEM and EDS mapping of TFEs.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Govindarajan, D., Palaniyandy, N., Chinnakutti, K.K. et al. Sputter-Deposited Binder-Free Nanopyramidal Cr/γ-Mo2N TFEs for High-Performance Supercapacitors. Nanoscale Res Lett 17, 65 (2022). https://doi.org/10.1186/s11671-022-03704-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11671-022-03704-5