Abstract
In recent years, silver halide (AgX, X = Cl, Br, I)-based photocatalytic materials have received increasing research attention owing to their excellent visible-light-driven photocatalytic performance for applications in organic pollutant degradation, HER, OER, and biomedical engineering. Ag as a noble metal has a surface plasma effect and can form Schottky junctions with AgX, which significantly promotes electron transport and increases photocatalytic efficiency. Therefore, Ag/AgX can reduce the recombination rate of electrons and holes more than pure AgX, leading to using AgX as a photocatalytic material in biomedical applications. The use of AgX-based materials in photocatalytic fields can be classified into three categories: AgX (Ag/AgX), AgX composites, and supported AgX materials. In this review, we introduce recent developments made in biomedical applications and biosensing diagnostics of AgX (Ag/AgX) photocatalytic materials. In addition, this review also discusses the photocatalytic mechanism and applications of AgX (Ag/AgX) and supported AgX materials.
Similar content being viewed by others
Introduction
Among various types of inorganic nanoparticles (NPs), silver NPs (AgNPs), due to their unique chemical, physical, and biological properties, have received a lot of attention for biomedical and bioanalytical applications [1,2,3,4,5]. For example, AgNPs are well known for their highly effective antimicrobial activities [2, 4, 6, 7] both in solutions and in solids. In addition, AgNPs have been widely used in several areas, such as sterilization [8,9,10], iconography, [11,12,13] water treatment [14,15,16], food package [17,18,19], agriculture [20,21,22,23], medicine [24,25,26,27], photocatalysis [28, 29], biotechnology [30,31,32], cancer therapy [33,34,35,36,37], Raman spectroscopy [38,39,40], electrochemistry [41,42,43,44], and environmental monitoring [44,45,46,47].
AgNPs can be combined with other nanomaterials to improve their performance. For example, AgNPs can be reduced on some semiconductors (such as TiO2, ZnO, and WO3) to form the Schottky junction [33, 48,49,50]. AgNPs have also been combined with magnetic NPs [51], graphene oxide [52], and quantum dots for various applications [53].
Silver halides have been widely used as industrial catalysts and photosensitive materials [54,55,56,57,58,59,60,61], and the use of silver halides is discussed in the following part. Chemically, silver has a strong affinity with halides to form various AgX (X = Cl, Br, and I) materials. There are numerous methods to prepare Ag/AgX. For example, AgX is doped on the surface of AgNPs by exposure to light of appropriate wavelengths, high-temperature reduction, and reducing agents. Ag/AgX shows better activity and selectivity toward certain applications. Thus, Ag/AgX as a nanocomposite has been extensively researched in recent years [8, 54, 62,63,64,65,66,67,68].
Since silver chloride has very low solubility in water, it is often used in the laboratory to determine the silver content of samples. Another very important application of silver chloride in electrochemistry is to make silver/silver chloride reference electrodes [69]. AgBr has been applied in several areas [70], such as color-changing lenses, in which a very small amount of silver bromide and copper oxide were added to ordinary glass. AgBr has photosensitivity and is often used in photographic films. A thin layer of gelatin containing fine AgBr NPs is coated on printing paper. When light with different intensities hit the film, AgBr decomposition products of different degrees are produced on the film. The more AgBr breaks down, the more darkness on that part. At last, AgI can condense water vapor in the air and form rain. Thus, AgI has been used as a nucleating reagent to precipitate supercold clouds and induce artificial rain. Aside from the above traditional applications, the photosensitivity of AgX has been recently utilized in biomedical and bioanalytical applications [71,72,73].
Although AgX can be applied in numerous ways, there are still some challenges and limitations. For example, AgX responds to ultraviolet and visible light, but light in this wavelength range cannot penetrate most human tissues. When applied to clinical studies, the toxicity of AgX has not been thoroughly studied. In some photocatalytic systems, photocorrosion of AgX can occur. Over the last few decades, a large surge in research activities emerged for biological applications of nanomaterials. Among them, the use of AgX has been growing most rapidly. Most related works were published in the last 15 years. In this article, we review the current status of the field with a focus on anticancer, antibacterial applications, and bioanalytical sensors.
Photocatalytic Activities of AgX
Before reviewing examples of various applications, we first describe the mechanism of photocatalytic activities, which are the basis for the design of materials and their properties. The principle of photocatalysis is based on the redox activity of photocatalysts under light to achieve degrading pollutants, material synthesis, and chemical transformations. Normally, photocatalytic oxidation uses semiconductors as catalysts and light as energy to degrade organic matter into carbon dioxide and water, where advanced oxidation by TiO2 is a primary example [48, 56, 74,75,76].
AgX belongs to semiconductors, and the bandgaps of AgCl, AgBr, and AgI are 3.28, 2.89, and 2.22 eV, respectively [77]. Pure AgX as a semiconductor has a low photocatalytic efficiency under ambient light irradiation [78,79,80]. Nevertheless, Ag nano-dots can still swiftly grow on the surface of AgX even with weak light irradiation [8, 54, 65,66,67, 69], which generates Ag/AgX nanocomposites.
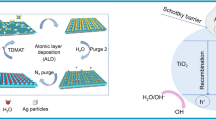
The photocatalytic mechanism of Ag/AgX nanocomposites is displayed in Fig. 1. The Ag metal as a conductor grown on the surface of AgX can easily generate electron–hole pairs, and then those electrons in the valance band can absorb the photons with energy equal to or higher than the bandgap energy. Those photo-generated charge carriers can only be deactivated by O2 or H2O.The first process is the photo-generated charge-mediate redox reactions, which include the oxidation of H2O to form OH· radicals, and the reduction of O2 to form reactive oxygen species (e.g., singlet oxygen, hydroxyl radical, superoxide, hydroperoxyl radical, and hydrogen peroxide) [81, 82]. This is the main process that can generate ROS. The second process is deactivation, which produces electron–hole pairs on the other side and recombines to obstruct redox reactions. Therefore, the second process decreases the efficiency of photocatalysis, which explains why pure AgNPs have a weak photocatalysis activity.
The photocatalytic mechanism of AgX and AgNPs. The AgX as a semiconductor absorbs photons under light irradiation, which generates photoelectron–hole pairs when the energy is greater than the bandgap. The photocarriers migrate to the surface under the action of the electric field or by diffusion motion. The electrons and holes migrate to the surface of the catalyst and react with the adsorbed material on the surface of the catalyst, respectively. Meanwhile, Ag as a conductor can promote electron transfer to the holes on AgX NPs
To inhibit the second process, that photo-generated charge recombination can be decreased by adding a semiconductor photocatalyst, such as AgX. AgX coated on AgNPs would naturally form a Schottky junction, which is supposed to trap the photo-generated charge carriers and then provide free electrons to minimize the recombination process [83,84,85,86,87]. Those electron–hole pairs generated by photo-excitation would migrate from AgNPs to the conduction band of AgX. In other words, these excess electrons in the semiconductor (AgX) side will migrate to holes in the metal side (Ag) through the Schottky junction.
Meanwhile, a large number of electrons generated by AgNPs can accelerate the process of photocatalysis, because of the localized surface plasmon resonance (LSPR) of AgNPs [57,58,59, 88,89,90]. Noble metal NPs, such as Au, Ag, Pt, and Cu, exhibit strong UV–Vis absorption due to their surface plasmon resonance (SPR). When AgX is decomposed into Ag0, the nanocomposite can demonstrate strong SPR and enhance the photocatalytic ability. As a result, plasmonic NPs can serve as an alternative type of sensitizer to enhance the visible light absorption of photocatalysts without the problem of degradation like organic sensitizers [91,92,93]. In 2008, Wang et al. fabricated Ag/AgCl plasmonic photocatalysts by an ion-exchange method [58], which triggered an upsurge in researching Ag/AgX plasmonic photocatalysts.
AgX for Cancer Therapy
Cancer has the biological characteristics of abnormal cell differentiation and proliferation, loss of control of growth, invasion, and metastasis. In 2020, an estimated 19.3 million new cancer cases and almost 10.0 million cancer deaths occurred worldwide. The most common cancers are esophagus, lung, stomach, breast, and cervical cancers. According to the analysis, the global cancer burden would increase by 28.4 million cases in 2040, a 47% rise from 2020. Meanwhile, a larger increase in developing (64% to 95%) countries will occur than in developed (32% to 56%) countries [94]. Therefore, nanotechnology in cancer treatment and diagnosis has gained popularity because it may have fewer side effects than traditional chemotherapeutics, and radiation therapy, which can generate cytotoxicity to normal cells as well [95,96,97,98,99,100,101]. To enhance the therapeutic effect and decreases the side effects, photodynamic therapy (PDT) is widely used in cancer treatment today [102,103,104,105,106,107,108,109,110].
AgX-based nanostructures have high biocompatibility, photocatalytic ability, and low toxicity, which have been regarded as attractive candidates to increase the clinical therapeutic effect of numerous kinds of cancer such as skin cancer and mouth cancer [33, 68, 96, 111]. Due to the limitation of effective wavelength, most current organic photosensitizers (less than 600 nm) cannot be used for tissues thicker than 0.1 cm since light cannot reach them. In addition, these organic photosensitizers are sometimes difficult to be excreted from the body. The wavelength range of AgX photosensitizers can be adjusted, and the application of a far infrared band can produce photothermal and photodynamic combined therapy. Since the particles can become smaller, they can be metabolized by the human body. In the following section, we review representative studies of Ag/AgX nanostructures in targeted drug delivery and controlled release systems.
Photodynamic Therapy
In PDT, the tumor site is irradiated under the light of specific wavelengths to activate photosensitive drugs, selectively concentrate them in the tumor tissue, and trigger photochemical reactions to destroy the tumor. The superoxide disproportionation reaction occurs under light irradiation. Photosensitive drugs can generate ROS (hydroxyl free radicals and superoxide free radicals), which can produce cytotoxicity and kill tumor cells. PDT has the advantage of delivering precise and effective treatments with fewer side effects than traditional cancer therapies.
Wang et al. reported DNA-templated plasmonic Ag/AgCl nanostructures in 2013 [111], which have selective photocatalysis and photocatalytic ability for killing cancer cells. They introduced DNA-programmable synthesis of ∼20 nm Ag/AgCl nanostructures by short-term (5 min) UV irradiation as shown in Fig. 2A, yielding monodispersed AgNPs in the presence of DNA. Moreover, these optimal DNA-encapsulated structures show DNA sequence-specific sizes down to less than 40 nm with an Ag/AgCl composition ratio of 2: 1 that afforded a vastly increased surface area and higher photocatalytic activity. The cell counting kit-8 (CCK-8) cytotoxicity assay showed a decrease of around 75% in the viability of the photocatalyst-treated HeLa cells with 532 nm light irradiation for 8 min (Fig. 2B).

Adapted from Ref. [111] with permission. Copyright (2013), Journal of Materials Chemistry B
A Illustration of the plasmonic Ag/AgCl nanostructure-based photocatalytic reaction mechanism. The DNA capping makes silver nanostructures negatively charged. B A comparison of viability among HeLa cells incubated with (+) and without (−) the Ag/AgCl nanostructures in PBS after photo-irradiation for 8 min.
In 2011, Seo’s group reported that serum protein-adsorbed Ag/AgBr/TiO2 NPs have photocytotoxicity in vitro and in vivo under visible light [112]. In this case, both Ag/AgBr and TiO2 had photocatalytic activities. The authors prepared Ag/AgBr/TiO2 by the deposition–precipitation method and found that they appeared to be well internalized by human carcinoma cells. As shown in Fig. 3A, the authors tested two types of cancer cells including human cervical HeLa and skin A431 cancer cells after incubation with and without the Ag/AgBr/TiO2 and subsequent exposure to visible light from a halogen lamp. Fluorescence images were taken to evaluate the labeled mitochondria activity, and the results suggested that ROS triggered the photo-destruction of the cancer cells. After applying the halogen light illumination for 50–250 min and ∼8 ppm (μg/mL) of the photocatalytic Ag/AgBr/TiO2, the authors observed a 40–60% decrease in cell viability (Fig. 3B). Finally, the Ag/AgBr/TiO2 was found to eliminate xenograft tumors significantly by irradiating visible light in vivo for 10 min.

Adapted from Ref. [112] with permission. Copyright (2011), Journal of Hazardous Materials
A Optical inverted phase-contrast microscopy images for the viability of HeLa cells treated with Ag/AgBr/TiO2 NPs incubated for longer than 24 h. Viability of HeLa cells as a function of halogen light illumination time. B The trypan blue exclusion test. The cells were treated with different Ag/AgBr/TiO2 NPs concentrations in the range of 2.0, 5.0, 8.0, and 14.0 ppm.
The above two examples had either DNA or TiO2 on the surface of Ag/AgX. In 2021, Zhang et al. reported that naked Ag/AgCl NPs can be applied for photocatalytic cancer treatment [68]. In this work, the authors synthesized biocompatible and surfactant-free Ag/AgCl NPs to investigate the photocatalytic efficacy. AgCl can be oxidized by FeCl3 on the surface of AgNPs, which naturally formed a Schottky junction. Thus, Ag as a co-catalyst can easily trap the photo-generated charge carriers and then provide free electrons, which would decrease the influence of the recombination process and increase photocatalytic efficacy. The photocatalytic efficacy of the Ag/AgCl NPs eliminated over 75% of HeLa cells under 30 min of light irradiation (Fig. 4A). Besides that, light irradiation stimulated the SPR of the AgNPs, which can enhance visible light absorption by the material. Compared to the pure AgNPs, the AgCl-coated AgNPs had a broader range of working wavelengths and higher photocatalytic efficacy (Fig. 4B). Moreover, it has less dark cytotoxicity than the pure AgCl NPs.

Adapted from Ref. [68] with permission. Copyright (2022), Chemical Engineering Journal
A The cell viability of Ag and different ratios of AgCl at Ag treated HeLa cells in a dark environment. B The comparison of HeLa cell viability treated by Ag and Ag/AgCl with or without simulated sunlight.
Cancer Cell Detection
An early cancer diagnosis is critical for the treatment and survival of cancer patients. Its purpose is to detect and treat early, and to reduce the physical, mental and economic burden of patients. Early cancer physical examination methods include a series of new cancer physical examination methods such as blood drop detection, genetic testing, nano-detection, and imaging [113, 114]. Laboratory tests can be used to analyze tumor markers, which substances are higher than normal levels found in the blood, urine, or tissues of some cancer patients. Imaging tests used to diagnose cancer may include computed tomography (CT) scans, bone scans, magnetic resonance imaging (MRI), positron emission tomography (PET) scans, ultrasound, and X-rays.
The methods for cancer cell detection can be conducted in two simple approaches. The first is that Ag/AgX nanomaterials can be composited with specific aptamers for cancer cells or folic acid (FA). Then, tumor-specific antigens or folate receptors will attract and bind the nanomaterials on those tumor cells. After that, colorless TMB is added in vitro and in vivo, which can be oxidized by Ag/AgX nanomaterials under light irradiation to blue-colored oxTMB, which can stain the tumor area. The second approach takes advantage of a high level of glutathione (GHS) in the tumor microenvironment, which has been generally regarded as a characteristic of cancerous tissues. AgBr nanomaterials can react with glutathione (GHS) to form AgNPs, which have a strong photoacoustic (PA) signal in the NIR-II region.
Silver halide has excellent imaging capability based on its physical property, which can be used in cancer detection. In 2014, Wang et al. reported a series of chitosan (CS) modified AgX NPs, which had dual-responsive enzyme mimetic activities [115]. In the presence of H2O2, the CS/AgX NPs were able to oxidize various colorimetric dyes, namely, peroxidase-like activity. Upon photoactivation, CS-AgX NPs could also oxidize the typical substrates in the absence of H2O2. Thus, the CS-modified silver halide was found to have dual-responsive enzyme mimetic activities. Based on these findings, the CS-AgI was regarded as a cost-effective, rapid, and highly sensitive colorimetric assay to detect cancer cells under light irradiation. The detection limit of the method for MDA-MB-231 was estimated to be as low as 100 cells, which was much lower than that reported by the method using peroxidase mimicking nanozymes based on other nanomaterials [116, 117].
Folic acid (FA), one of the best-characterized biological ligands, is widely employed to target tumors or cancer cells because folate receptors are usually overexpressed on the membrane of human cancer cells [118, 119]. FA-CS-AgI conjugates were synthesized by chemically coupling FA to CS via the formation of an amide bond between the amine groups of CS and the carboxyl groups of FA. As Fig. 5 shows, cells were first fixed on a 96-well plate. Then the FA-AgI conjugate was added to the folate receptor, which is a simple binding reaction and no antibodies were involved. Then, taking advantage of the photo-oxidase-like activity of AgI, TMB was added as a substrate. Upon irradiation with visible light (λ ≥ 420 nm), blue-colored TMB oxidation products were formed in the presence of tumor cells.

Adapted from Ref. [115] with permission. Copyright (2014) ACS Publications
Proposed detection process using FA-CS-AgI.
In 2017, Zhang’s group reported a Cu2+-doped Ag–AgI nanocomposite (Cu2+/Ag–AgI), which was designed for photosensitive colorimetric immunoassay for tumor marker detection [120]. Figure 6A clearly shows that it was designed as a highly photosensitive colorimetric immunoassay for carcinoembryonic antigen (CEA) detection based on the Cu2+/Ag–AgI. The Cu2+/Ag–AgI can oxidize TMB, which was synthesized by an impregnation method. After that, they achieved the immune-complex built on the surface of a magnetic bead by utilizing Cu2+/Ag–AgI as labels based on a sandwich-type immunoassay. The blue color of TMBox was obtained under visible irradiation and ultraviolet spectrum scanning, which showed increased absorbance values with increasing CEA concentrations. Above all, the developed colorimetric immunoassay exhibited good selectivity, repeatability, stability, and possible applications in the real serum sample analysis.

Adapted from Ref. [120] with permission. Copyright (2017), Talanta. Copyright (2018), Journal of Materials Chemistry B
A The schematic illustration of oxidation of TMB by Cu2+/Ag–AgI B Photo-generated electron–hole transfer mechanism of C3N4–AgX in the presence of 0.1 M K4[Fe(CN)6].
In 2018, Mazhabi et al. reported a C3N4–AgI/ITO photoelectrode for specific cervical cancer HeLa cell recognition [121]. They claimed that the C3N4–AgI/ITO photoelectrode as a label-free aptamer-based cytosensor can be applied in cancer diagnostics. As a new nanomaterial, the C3N4–AgI/ITO photoelectrode has both photoactive and bio-recognition features, which perfectly satisfied the requirements of photoelectrochemical (PEC) biosensors for cancer cell detection. In this study, the authors designed a novel label-free PEC aptamer-based cytosensor for the specific detection of cancer cells such as HeLa cells by using water-dispersible g-C3N4–AgI nanocomposites as visible light-sensitive materials and an anti-CEM/PTK7 aptamer as the bio-recognition element. As shown in Fig. 6B, when a suitable amount of AgI NPs was doped in the two-dimensional graphite-like carbon nitride nano-sheets (g-C3N4 NSs), the visible light photocurrent response could be significantly improved. The PEC response of the as-prepared biosensor based on the g-C3N4–AgI/ITO photoelectrode was linearly proportional to the relevant cancer cells such as HeLa cells in concentrations ranging from 10 to 106 cells per mL with a limit of detection of 5 cells per mL. In addition, the g-C3N4–AgI/ITO photoelectrode and the fabricated cytosensor exhibited long-term stability, good reproducibility, excellent selectivity, and high sensitivity, demonstrating the successful conjugation of g-C3N4–AgI NSs with the aptamer and targeting cancer cells in the high-performance PEC cytosensor.
In 2021, Cui et al. reported that AgBr@PLGA nanocrystals can be applied in ultrahigh-sensitive and tumor-specific photoacoustography in the near-infrared NIR-II region [122]. The highly up-regulated glutathione (GSH) concentration in the tumor microenvironment is generally identified to be an effective endogenous characteristic of cancerous tissues. Therein, an ultrahigh-sensitive and tumor-specific photoacoustography technique in the NIR-II region based on optical writing and redox-responsive chromogenic graphic fixing was developed by introducing a self-synthesized photosensitive silver bromide modified with poly lactic-co-glycolic acid (AgBr@PLGA) nanocrystals. After optically triggered by external light, the NIR-transparent AgBr@PLGA nanocrystals can be reduced by the tumor-abundant GSH into strongly absorbing AgNPs, significantly boosting the “turn-on” photoacoustic (PA) signal in the NIR-II region. As shown in Fig. 7, the tumor area can be graphically fixed and developed in the photoacoustography. Experiments on both in vitro phantoms and in vivo mouse models demonstrated that the tumor area was specifically identified by the photoacoustography with the background signals effectively suppressed by dynamically modulating the exposure time.

Adapted from Ref. [122] with permission. Copyright (2021), ACS Publications
Synthesis process of the prepared AgBr@PLGA NCs and the schematic illustration that tumor area is graphically fixed via redox reaction where the NIR-transparent AgBr@PLGA NCs can be reduced by the tumor-abundant GSH into strongly absorbing silver NPs.
Mantri et al. reported that iodide-doped precious metal NPs, such as Ag@AuNR, can be used to measure oxidative stress in vivo via photoacoustic imaging [123]. Since the accumulation of reactive oxygen and nitrogen species (RONS) can directly influence cell damage and cell death, the measurement of RONS is important for cancer detection. However, both reactive oxygen and nitrogen species are short-lived and exist only in trace amount in vivo (biologically RONS levels are in the nM–μM scale). Therefore, they used halide doping to tune the electrochemical properties of gold-core/silver-shell NPs to match the oxidation potential of RONS. The proposed mechanism is shown in Fig. 8. In this work, the authors provided the synthesis, characterization, and application of these AgI-coated gold nanorods (AgI/AuNR) and discussed that halide doping lowered the reduction potential of Ag, which resulted in a 1000-fold increase in H2O2 and 100,000-fold increase in ONOO− sensitivity. The AgI/AuNR system also etched 45-fold faster than the undoped Ag/AuNR.

Adapted from Ref. [123] with permission. Copyright (2020), Royal Society of Chemistry
Schematic of shell optimization. Schematic representation of the current work. Iodide doping results in biologically relevant [nM–μM] sensitivity to RONS.
AgX-Based Antibacterial Nanomaterials
Bacteria are an important cause of disease and infection. Some microorganisms such as golden staphylococci may cause serious human tissue damage. Thus, the preparation of antibacterial materials has caused widespread interest [6, 124, 125]. One of the well-known applications of AgNPs is antimicrobial. Aside from pure AgNPs, using its AgX hybrid materials has also been reported.
Recently, Ag@AgCl as a highly efficient photocatalyst was applied to the photocatalytic degradation of pollutants and organic synthesis products [63, 126,127,128]. Since the charge hole combination rate of the AgCl is low under visible light, and the active oxygen species, such as hydroxyl radicals, are relatively high in concentration and are applied to the preparation of antibacterial materials.
In 2017, Mao et al. reported that Ag/Ag@AgCl/ZnO hybrid nanostructures exhibited a high antibacterial efficiency against both Escherichia coli (E. coli) and Staphylococcus aureus under visible light irradiation (Fig. 9) [8]. In their study, the Ag/Ag@AgCl nanostructures enhanced the antibacterial activity of ZnO. This hydrogel system killed 95.95% of E. coli and 98.49% of S. aureus under visible light irradiation after 20 min. In addition, this system provided about 90% Zn2+ release in the acidic environment within 3 days, but 10% Zn2+ release occurred in the neutral environment within 21 days. The results showed that the release of Ag+ and Zn2+ stimulated the immune function to produce a large number of white blood cells and neutrophils, which resulted in the production of antibacterial effects.

Adapted from Ref. [8] with permission. Copyright (2017) ACS Nano
Mechanism of Ag/Ag@AgCl/ZnO antibacterial.
Silver bromide (AgBr) is an n-type photosensitive semiconductor with a 2.6 eV bandgap [61]. Ag/AgBr NPs are applied to the disinfection of bacteria because of the plasma effect and high efficiency charge molecular efficiency under light irradiation [59, 129].
In 2012, Padervand et al. evaluated the photocatalytic activities of zeolite-based Ag/AgBr and Ag/AgBr/TiO2 photocatalysts for the inactivation of E. coli [9]. In their study, the Ag/AgBr/zeolite and Ag/AgBr/TiO2/zeolite had a high activity and decreased E. coli concentration most, but the zeolite and TiO2/zeolite had a lower antibacterial activity (Fig. 10A). After the visible light illumination, the electron–hole pairs of Ag/AgBr/TiO2/zeolite were generated. The electrons of AgBr conduction band can be transferred to TiO2 conduction band, which is below the AgBr conduction band. This property increased the photoreaction efficiency (Fig. 10B).

Adapted from Ref. [9] with permission. Copyright (2012) Materials Science in Semiconductor Processing
A E. coli inactivation curve over the composites at the visible light (C0 = 1 × 107, 30 mL, 12 mg of catalyst). B Electrons transfer from AgBr conduction band to the lower position TiO2 conduction band and delay the electron–hole recombination.
In 2019, Zhang et al. reported that AgBr/AgVO3 photocatalysts exhibited enhanced antibacterial efficiency compared to pure AgBr and pure AgVO3 under visible light irradiation [130]. More than 99.9970% of E. coli, S. aureus, and P. aeruginosa cells were killed by the photocatalysis of 0.5AgBr/AgVO3 after 30 min. Overall, the 0.5AgBr/AgVO3 heterojunction photocatalyst had excellent photocatalytic antibacterial performance (Fig. 11).

Adapted from Ref. [130] with permission. The Royal Society of Chemistry
A P. aeruginosa survival curves in the antibacterial experiments, B photocatalytic antibacterial rates of E. coli, S. aureus and P. aeruginosa with 0.5AgBr/AgVO3 for 30 min, and the survival pictures of P. aeruginosa colonies in the presence of different photocatalysts. C Copyright (2019).
In 2020, Lin et al. reported a three-dimensional structured Ag-AgBr/BiVO4/graphene aerogel (Ag-AgBr/BiVO4/GA) via a hydrothermal and freeze-drying process [131]. In their study, the Ag-AgBr/BiVO4/GA exhibited a good disinfection performance toward E. coli (100% removal rate in 24 min). The proposed photocatalytic mechanism of inactivating E. coli/S. aureus by Ag-AgBr/BiVO4/GA is presented in Fig. 12. Ag-AgBr/BiVO4/GA was activated to excited states under visible light, and electrons in the valence band of AgBr and BiVO4 were excited to the conduction band (CB). Thus, both two semiconductors can generate electrons and holes. The existence of GA served as an electro-reservoir. Subsequently, the electrons formed in the CB of BiVO4 moved to the AgNPs, and the holes transferred from the VB of the AgBr, which caused the accumulation of electrons in the CB of AgBr and holes in the VB of BiVO4. Finally, the electrons in the CB reacted with O2 or H2O to generate active oxygen species, which can oxidize live bacteria into inactive bacteria.

Adapted from Ref. [131] with permission. Copyright (2020) Colloids and Surfaces A
The proposed tentative mechanism for photocatalytic disinfection of Ag-AgBr/ BiVO4/GA composite under visible light irradiation.
Not only that but AgI can also be used as antibacterial material. In 2012, Ghosh et al. synthesized a core@shell structure consisting of AgNPs and AgI in an agarose matrix (Ag@AgI/agarose), which was an efficient antibacterial agent [7]. In situ synthesis of the catalyst was achieved by adding a low concentration of KI solution gradually. The authors speculated that the AgI thin layer was first formed by AgNPs, and then the AgI layer gradually became thick with the addition of the KI solution (Fig. 13A). Antibacterial studies including repetitive cycles were carried out on Gram-negative E. coli and Gram-positive Staphylococcus aureus bacteria in saline water, both in dark and under visible light. The Ag@AgI/agarose could be reused in the antibacterial experiment (Fig. 13B).

Adapted from Ref. [7] with permission. Copyright (2012) Langmuir
A Schematic illustration of the stepwise formation of core@shell structure of Ag@AgI in agarose matrix. B Viable cell count test by nutrient agar plating: percentage survival bacteria versus contact time for (a) E. coli and (b) S. aureus in the dark, and (c) E. coli and (d) S. aureus in the presence of light.
AgX-Based Biosensors
A biosensor is a target-specific biomolecule coupled with a signal transduction unit that can be used to identify target analytes with high sensitivity and specificity. AgX has been used to prepare a high sensitivity biosensor, which can detect genes, urine, and blood in humans [132,133,134,135]. A recent development is the detection of glucose. Glucose oxidase can oxidize glucose to produce gluconolactone and the reduced enzyme. Subsequently, the reduced enzyme transfers electrons to molecular oxygen in the body's biological fluid to produce hydrogen peroxide (H2O2) [136, 137]. Ag/AgX as electrode materials were applied in several biosensor systems because it has good reversibility and its electrode potential is relatively stable [138].
The stability of biosensors is significant to real-world applications. Table 1 summarizes the durability of those Ag/AgX nanomaterials. Most of them used degradation efficiency and control of the light on/off states to test the stability of biosensors.
In 2021, Chen’s group reported a new nanomaterial, Ag@AgCl photocatalyst loaded on 3D graphene/PANI hydrogels that can be used for in situ SERS monitoring [139]. With silver halides AgX (X = Cl, Br, I) and the surface plasmon resonance (SPR) of metallic Ag, heterojunctions of Ag@AgX can exhibit high stability and highly efficient utilization of visible light. The authors synthesized GH/PANI/Ag@AgCl nanocomposites by reacting Ag+ ions with the Cl− in the HCl-doped PANI in 3D GH/PANI and the subsequent photo-assisted reduction. These new nanomaterials have enhanced adsorption-photocatalytic degradation. Due to combining AgNPs with graphene and PANI, it can be applied for real-time SERS detection. Hence, graphene/PANI composites exhibited enhanced electrical conductivity and electrochemical stability compared with bare PANI, which has been widely applied in biosensors because of its optical, electronic, and electrochemical features.
2016, Chang et al. used AgI/TiO2 hybrid material as a label to produce a color signal with a visible optic excitation for quantitative analysis of chloramphenicol (CAP) (Fig. 14). [48] The enzyme-like catalytic nanomaterials are also known as nanozymes [143,144,145,146]. Using a competitive immunoassay with CS-AgI/TiO2 labeled CAP antibodies as tags, detection of CAP was achieved on the surface of CAP-BSA modified magnetic bead. In this reaction, the participation of hydrogen peroxide was not required [48]. The combination of AgI and TiO2 effectively reduced the composite of photocarriers and electrons, which greatly enhanced the ability of solution color (Fig. 14A,B). This allowed the immunosensor sensor to have better sensitivity and a low detection limit of 0.03 nM.

Adapted from Ref. [48] with permission. Copyright (2017) Biosensors and Bioelectronics
A The UV–Vis spectrums of (a) TiO2 + TMB, (b) AgI + TMB, and (c) CS-AgI/TiO2 + TMB under visible light illumination [the inset is the photographs of a, b, c]; B The UV–Vis spectrums of (a) Fe3O4 + TMB, (b) CS-AgI/TiO2 + TMB, and (c) Fe3O4 + CS–AgI/TiO2 + TMB; C the UV–Vis absorbance of different immunosensors for 0.5 nM CAP (a) anti-CAP-CS-TiO2/CAP-BSA/MB, (b) anti-CAP-CS-AgI/CAP-BSA/MB, and (c) anti-CAP-CS-AgI/TiO2/CAP-BSA/MB [the inset is ΔA of a, b, c for 0.5 nM CAP]; The effect of D incubation time of antigen–antibody, E pH of ABS, and F concentration of TMB.
In 2020, Hashemi et al. reported that AgBr NPs with the polymeric structure of PT could sense glucose through a real-time and precise approach [138]. In their system, PT-AgBr was modified cubic, anorthic or hybrid cubic–anorthic morphologies (Fig. 15A). They confirmed the successful formation of AgBr, PT and PT-AgBr by diverse characterizations, for example, X-ray diffractogram, zeta potential, and FESEM. PT has a conductive polymeric structure. So, its integration with AgBr can improve its electrocatalytic performance, Further, glucose detection can be accurately performed (Fig. 15B), and the PT-AgBr showed zeta potential and electrophoretic mobility of 16.7 mV/0.000129 cm2/Vs, respectively.

Adapted from Ref. [138] with permission. Copyright (2020) European Polymer Journal
A FESEM images of (e–h) PT-AgBr B Non-enzymatic biosensing mechanism of glucose via the developed platform within the human blood plasma.
In 2021, Zhu et al. reported that 3D Z-scheme AgI/Ag/BiOI heterojunction for highly sensitive determination of CAP, thereby fabricating a “signal-on” PEC aptasensor [140]. In their study, the AgI/Ag/BiOI photocatalyst was prepared by an ion-exchange and photo-irradiation method, which improved the photocurrents by facilitating the spatial separation of the photo-generated charge carriers. The response of the PEC aptasensor was linearly correlated between the photocurrents and the logarithm of the CAP concentrations, which had the advantages of being sensitive, simple and stable (Fig. 16).

Adapted from Ref. [140] with permission. Copyright (2021) Biosensors and Bioelectronics
Photocurrents A of the developed PEC aptasensor with different CAP concentrations (curves a–h: 0–250 nM). Calibration curve (B). Selectivity (C) toward 200 nM CAP with equal amount of the interfering substances (i.e., kanamycin, lincomycin, tetracycline, ciprofloxacin, and ampicillin), and their mixture. Stability (D) of the as-fabricated PEC aptasensor.
In 2019, Hao et al. reported AgBr NPs-anchored 3D nitrogen-doped graphene hydrogel (3DNGH) nanocomposites with a large surface by hydrothermal method (Fig. 17) [147]. With a porous structure, 3DNGH had a large surface area that can favor the immobilization of AgBr and luminol, where in turn, luminol and hydrogen peroxide can be in close contact. They fabricated all-solid-state luminol electrochemiluminescence E. coli aptasensors, which showed great performance. So, the potential for the luminol/AgBr/3DNGH nanocomposite for E. coli detection in biomedical and environmental analysis was developed.

Adapted from Ref. [147] with permission. Copyright (2017) Biosensors and Bioelectronics
The schematic presentation for the ECL Escherichia coli biosensor fabricated with luminol/AgBr/3DNGH.
Summary and Perspectives
In this article, we reviewed the biomedical and bioanalytical applications of AgX. Silver halide has been widely used in industry as electrodes (Ag/AgCl), imaging materials (AgBr), and artificial rainfall (AgI).
However, AgX NPs as photocatalytic materials have not been intensively studied because they decompose to produce AgNPs under visible light. Through the combination of silver NPs and silver halide, the decomposition rate of silver halide can be significantly reduced. The principle is that silver halide, as a semiconductor inorganic photocatalyst, will generate photo-generated carriers and generate electrons and holes under visible light irradiation. However, the rate of this process is low because electrons and holes will combine, reducing the occurrence of oxidation reactions. Therefore, the silver elementary substance can be doped into silver halide, because elemental silver, as a precious metal, can produce a surface plasmon effect and promote electron transport. One of the drawbacks is the potential photofatigue effect during long-term irradiation, which may result in a gradual loss of efficiency. The solution is that AgNPs and AgX form a Schottky junction, which reduces the contact between electrons and holes, thereby promoting photocatalytic efficiency and extending the working life. Based on the above discussion, it is possible to apply Ag/AgX photocatalysts in the fields of cancer treatment, cancer diagnosis, antibacterial, wound healing, and biosensor.
The applications of Ag/AgX in medicine are only at the proof-of-concept stage because the working wavelength for Ag/AgX nanomaterials was almost exclusively in the UV/visible region, and the short-wavelength light can suffer from phototoxicity and poor tissue penetration. To solve this problem, a near-infrared (NIR) light featuring lower toxicity and deeper tissue penetration has more advantages in biological applications. By doping heterojunctions and other methods, the range of photocatalytic wavelengths can be increased, and the energy bandgap can be reduced so that the silver halide nanomaterial can absorb more energy in a broad wavelength range. At the same time, AgX materials also have certain limitations. For example, simple AgX is susceptible to decomposition under strong light, and AgNPs produced by the decomposition have cytotoxicity to human tissues and organs. Therefore, further research on the application of silver halide surface plasma materials in the field of photocatalysis is indispensable.
Availability of data and material
The datasets used or analyzed during the study are available from the related journals and the corresponding author upon reasonable request.
References
Christensen L, Vivekanandhan S, Misra M, Kumar Mohanty A (2011) Biosynthesis of silver nanoparticles using Murraya koenigii (curry leaf): an investigation on the effect of broth concentration in reduction mechanism and particle size. Adv Mater Lett 2(6):429–434
Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramírez JT et al (2005) The bactericidal effect of silver nanoparticles. Nanotechnology 16(10):2346
Naik RR, Stringer SJ, Agarwal G, Jones SE, Stone MO (2002) Biomimetic synthesis and patterning of silver nanoparticles. Nat Mater 1(3):169–172
Rai M, Yadav A, Gade A (2009) Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv 27(1):76–83
Kim C, Jeon HS, Eom T, Jee MS, Kim H, Friend CM et al (2015) Achieving selective and efficient electrocatalytic activity for CO2 reduction using immobilized silver nanoparticles. J Am Chem Soc 137(43):13844–13850
Brogden KA (2005) Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol 3(3):238–250
Ghosh S, Saraswathi A, Indi SS, Hoti SL, Vasan HN (2012) Ag@AgI, core@shell structure in agarose matrix as hybrid: synthesis, characterization, and antimicrobial activity. Langmuir 28(22):8550–8561
Mao C, Xiang Y, Liu X, Cui Z, Yang X, Yeung KWK et al (2017) Photo-inspired antibacterial activity and wound healing acceleration by hydrogel embedded with Ag/Ag@AgCl/ZnO nanostructures. ACS Nano 11(9):9010–9021
Padervand M, Elahifard MR, Meidanshahi RV, Ghasemi S, Haghighi S, Gholami MR (2012) Investigation of the antibacterial and photocatalytic properties of the zeolitic nanosized AgBr/TiO2 composites. Mater Sci Semicond Process 15(1):73–79
Setterington EB, Alocilja EC (2012) Electrochemical biosensor for rapid and sensitive detection of magnetically extracted bacterial pathogens. Biosensors 2(1):15–31
Du P, Niu Q, Chen J, Chen Y, Zhao J, Lu X (2020) “Switch-On” fluorescence detection of glucose with high specificity and sensitivity based on silver nanoparticles supported on porphyrin metal–organic frameworks. Anal Chem 92(11):7980–7986
Dwivedi P, Narvi SS, Tewari RP (2012) Rudraksha assisted generation of silver nanoparticles for integrated application in the biomedical landscape. Int J Green Nanotechnol 4(3):248–261
Shang L, Qin C, Jin L, Wang L, Dong S (2009) Turn-on fluorescent detection of cyanide based on the inner filter effect of silver nanoparticles. Analyst 134(7):1477–1482
Dankovich TA, Gray DG (2011) Bactericidal paper impregnated with silver nanoparticles for point-of-use water treatment. Environ Sci Technol 45(5):1992–1998
Kallman EN, Oyanedel Craver VA, Smith JA (2011) Ceramic filters impregnated with silver nanoparticles for point-of-use water treatment in rural Guatemala. J Environ Eng 137(6):407–415
Morsi RE, Alsabagh AM, Nasr SA, Zaki MM (2017) Multifunctional nanocomposites of chitosan, silver nanoparticles, copper nanoparticles and carbon nanotubes for water treatment: antimicrobial characteristics. Int J Biol Macromol 97:264–269
Carbone M, Donia DT, Sabbatella G, Antiochia R (2016) Silver nanoparticles in polymeric matrices for fresh food packaging. J King Saud Univ Sci 28(4):273–279
De Moura MR, Mattoso LH, Zucolotto V (2012) Development of cellulose-based bactericidal nanocomposites containing silver nanoparticles and their use as active food packaging. J Food Eng 109(3):520–524
Kumar S, Shukla A, Baul PP, Mitra A, Halder D (2018) Biodegradable hybrid nanocomposites of chitosan/gelatin and silver nanoparticles for active food packaging applications. Food Packag Shelf Life 16:178–184
Chhipa H (2017) Nanofertilizers and nanopesticides for agriculture. Environ Chem Lett 15(1):15–22
Kashyap PL, Kumar S, Srivastava AK, Sharma AK (2013) Myconanotechnology in agriculture: a perspective. World J Microbiol Biotechnol 29(2):191–207
Mishra S, Singh H (2015) Biosynthesized silver nanoparticles as a nanoweapon against phytopathogens: exploring their scope and potential in agriculture. Appl Microbiol Biotechnol 99(3):1097–1107
Sujatha J, Suriya P, Rajeshkumar S (2017) Biosynthesis and characterization of silver nanoparticles by actinomycetes isolated from agriculture field and its application on antimicrobial activity. Res J Pharm Technol 10(6):1963–1968
Alarcon E, Vulesevic B, Argawal A, Ross A, Bejjani P, Podrebarac J et al (2016) Coloured cornea replacements with anti-infective properties: expanding the safe use of silver nanoparticles in regenerative medicine. Nanoscale 8(12):6484–6489
Shenava A, Sharma SM, Shetty V, Shenoy S (2015) Silver nanoparticles: a boon in clinical medicine. J Oral Res Rev 7(1):35
Singh M, Singh S, Prasad S, Gambhir I (2008) Nanotechnology in medicine and antibacterial effect of silver nanoparticles. Dig J Nanomater Biostruct 3(3):115–122
Wong KK, Liu X (2010) Silver nanoparticles—The real “silver bullet” in clinical medicine? MedChemComm 1(2):125–131
Awazu K, Fujimaki M, Rockstuhl C, Tominaga J, Murakami H, Ohki Y et al (2008) A plasmonic photocatalyst consisting of silver nanoparticles embedded in titanium dioxide. J Am Chem Soc 130(5):1676–1680
Sarina S, Waclawik ER, Zhu H (2013) Photocatalysis on supported gold and silver nanoparticles under ultraviolet and visible light irradiation. Green Chem 15(7):1814–1833
Khatoon N, Mazumder JA, Sardar M (2017) Biotechnological applications of green synthesized silver nanoparticles. J Nanosci Curr Res 2(107):2572–0813
Sheikh FA, Barakat NA, Kanjwal MA, Chaudhari AA, Jung I-H, Lee JH et al (2009) Electrospun antimicrobial polyurethane nanofibers containing silver nanoparticles for biotechnological applications. Macromol Res 17(9):688–696
Sheikh FA, Barakat NA, Kanjwal MA, Jeon SH, Kang HS, Kim HY (2010) Self synthesize of silver nanoparticles in/on polyurethane nanofibers: nano-biotechnological approach. J Appl Polym Sci 115(6):3189–3198
AbuMousa RA, Baig U, Gondal MA, AlSalhi MS, Alqahtani FY, Akhtar S et al (2018) Photo-catalytic killing of HeLa cancer cells using facile synthesized pure and Ag loaded WO3 nanoparticles. Sci Rep 8(1):15224
Dhanalekshmi KI, Magesan P, Sangeetha K, Zhang X, Jayamoorthy K, Srinivasan N (2019) Preparation and characterization of core-shell type Ag@SiO nanoparticles for photodynamic cancer therapy. Photodiagn Photodyn Ther 28:324–329
Maji SK, Kim DH (2018) AgInS2-coated upconversion nanoparticle as a photocatalyst for near-infrared light-activated photodynamic therapy of cancer cells. ACS Appl Bio Mater 1(5):1628–1638
Wang Q, Wang C, Wang X, Zhang Y, Wu Y, Dong C et al (2019) Construction of CPs@MnO-AgNPs as a multifunctional nanosensor for glutathione sensing and cancer theranostics. Nanoscale 11(40):18845–18853
Yang L, Kim TH, Cho HY, Luo J, Lee JM, Chueng STD, et al (2020) Hybrid graphene-gold nanoparticle-based nucleic acid conjugates for cancer-specific multimodal imaging and combined therapeutics. Adv Funct Mater
Chattopadhyay S, Lo HC, Hsu CH, Chen LC, Chen KH (2005) Surface-enhanced Raman spectroscopy using self-assembled silver nanoparticles on silicon nanotips. Chem Mater 17(3):553–559
Emory SR, Nie S (1997) Near-field surface-enhanced Raman spectroscopy on single silver nanoparticles. Anal Chem 69(14):2631–2635
Stamplecoskie KG, Scaiano JC, Tiwari VS, Anis H (2011) Optimal size of silver nanoparticles for surface-enhanced Raman spectroscopy. J Phys Chem C 115(5):1403–1409
Defnet PA, Anderson TJ, Zhang B (2020) Stochastic collision electrochemistry of single silver nanoparticles. Curr Opin Electrochem 22:129–135
Ivanova OS, Zamborini FP (2010) Size-dependent electrochemical oxidation of silver nanoparticles. J Am Chem Soc 132(1):70–72
Rodriguez-Sanchez L, Blanco MC, López-Quintela MA (2000) Electrochemical synthesis of silver nanoparticles. J Phys Chem B 104(41):9683–9688
Zhou YG, Rees NV, Compton RG (2011) The electrochemical detection and characterization of silver nanoparticles in aqueous solution. Angew Chem Int Ed 50(18):4219–4221
Patlolla AK, Berry A, May L, Tchounwou PB (2012) Genotoxicity of silver nanoparticles in Vicia faba: a pilot study on the environmental monitoring of nanoparticles. Int J Environ Res Public Health 9(5):1649–1662
Stensberg MC, Wei Q, McLamore ES, Porterfield DM, Wei A, Sepúlveda MS (2011) Toxicological studies on silver nanoparticles: challenges and opportunities in assessment, monitoring and imaging. Nanomedicine 6(5):879–898
Yu SJ, Yin YG, Liu JF (2013) Silver nanoparticles in the environment. Environ Sci Process Impacts 15(1):78–92
Chang H, Lv J, Zhang H, Zhang B, Wei W, Qiao Y (2017) Photoresponsive colorimetric immunoassay based on chitosan modified AgI/TiO2 heterojunction for highly sensitive chloramphenicol detection. Biosens Bioelectron 87:579–586
Zhu X, Liang X, Wang P, Dai Y, Huang B (2018) Porous Ag-ZnO microspheres as efficient photocatalyst for methane and ethylene oxidation: Insight into the role of Ag particles. Appl Surf Sci 456:493–500
Ziashahabi A, Prato M, Dang Z, Poursalehi R, Naseri N (2019) The effect of silver oxidation on the photocatalytic activity of Ag/ZnO hybrid plasmonic/metal-oxide nanostructures under visible light and in the dark. Sci Rep 9(1):11839
Li H, Jiang D, Huang Z, He K, Zeng G, Chen A et al (2019) Preparation of silver-nanoparticle-loaded magnetic biochar/poly(dopamine) composite as catalyst for reduction of organic dyes. J Colloid Interface Sci 555:460–469
Kumari S, Sharma P, Yadav S, Kumar J, Vij A, Rawat P et al (2020) A novel synthesis of the graphene oxide-silver (GO-Ag) nanocomposite for unique physiochemical applications. ACS Omega 5(10):5041–5047
Chen S, Quan Y, Yu YL, Wang JH (2017) Graphene quantum dot/silver nanoparticle hybrids with oxidase activities for antibacterial application. ACS Biomater Sci Eng 3(3):313–321
Cai B, Wang J, Gan S, Han D, Wu Z, Niu L (2014) A distinctive red Ag/AgCl photocatalyst with efficient photocatalytic oxidative and reductive activities. J Mater Chem A 2(15):5280–5286
Dong R, Tian B, Zeng C, Li T, Wang T, Zhang J (2012) Ecofriendly synthesis and photocatalytic activity of uniform cubic Ag@AgCl plasmonic photocatalyst. J Phys Chem C 117(1):213–220
Pratap Reddy M, Venugopal A, Subrahmanyam M (2007) Hydroxyapatite-supported Ag-TiO2 as Escherichia coli disinfection photocatalyst. Water Res 41(2):379–386
Wang P, Huang B, Lou Z, Zhang X, Qin X, Dai Y et al (2010) Synthesis of highly efficient Ag@ AgCl plasmonic photocatalysts with various structures. Chem Eur J 16(2):538–544
Wang P, Huang B, Qin X, Zhang X, Dai Y, Wei J et al (2008) Ag@AgCl: a highly efficient and stable photocatalyst active under visible light. Angew Chem Int Ed Engl 47(41):7931–7933
Wang P, Huang B, Zhang Q, Zhang X, Qin X, Dai Y et al (2010) Highly efficient visible light plasmonic photocatalyst Ag@ Ag (Br, I). Chem Eur J 16(33):10042–10047
Yan Q, Sun M, Yan T, Li M, Yan L, Wei D et al (2015) Fabrication of a heterostructured Ag/AgCl/Bi2MoO6 plasmonic photocatalyst with efficient visible light activity towards dyes. RSC Adv 5(22):17245–17252
Yang Y, Guo W, Guo Y, Zhao Y, Yuan X, Guo Y (2014) Fabrication of Z-scheme plasmonic photocatalyst Ag@ AgBr/g-C3N4 with enhanced visible-light photocatalytic activity. J Hazard Mater 271:150–159
Gamage McEvoy J, Bilodeau DA, Cui W, Zhang Z (2013) Visible-light-driven inactivation of Escherichia coli K-12 using an Ag/AgCl–activated carbon composite photocatalyst. J Photochem Photobiol A Chem 267:25–34
Wu YA, Li L, Li Z, Kinaci A, Chan MKY, Sun Y et al (2016) Visualizing redox dynamics of a single Ag/AgCl heterogeneous nanocatalyst at atomic resolution. ACS Nano 10(3):3738–3746
Zeng C, Hu Y, Guo Y, Zhang T, Dong F, Zhang Y et al (2016) Facile in situ self-sacrifice approach to ternary hierarchical architecture Ag/AgX (X = Cl, Br, I)/AgIO3 distinctively promoting visible-light photocatalysis with composition-dependent mechanism. ACS Sustain Chem Eng 4(6):3305–3315
Ghaly HA, El-Kalliny AS, Gad-Allah TA, Abd El-Sattar NEA, Souaya ER (2017) Stable plasmonic Ag/AgCl–polyaniline photoactive composite for degradation of organic contaminants under solar light. RSC Adv 7(21):12726–12736
Jin C, Liu X, Tan L, Cui Z, Yang X, Zheng Y et al (2018) Ag/AgBr-loaded mesoporous silica for rapid sterilization and promotion of wound healing. Biomater Sci 6(7):1735–1744
Tao S, Yang M, Chen H, Zhao S, Chen G (2018) Continuous synthesis of Ag/AgCl/ZnO composites using flow chemistry and photocatalytic application. Ind Eng Chem Res 57(9):3263–3273
Zhang X, Wang P, Meng W, Cui E, Zhang Q, Wang Z et al (2022) Photococatalytic anticancer performance of naked Ag/AgCl nanoparticles. Chem Eng J 428:131265
Brewer PJ, Leese RJ, Brown RJ (2012) An improved approach for fabricating Ag/AgCl reference electrodes. Electrochim Acta 71:252–257
Kang M, Deng Y, Oderinde O, Su F, Ma W, Yao F et al (2019) Sunlight-driven photochromic hydrogel based on silver bromide with antibacterial property and non-cytotoxicity. Chem Eng J 375:121994
Subbaiya R, Saravanan M, Priya AR, Shankar KR, Selvam M, Ovais M et al (2017) Biomimetic synthesis of silver nanoparticles from Streptomyces atrovirens and their potential anticancer activity against human breast cancer cells. IET Nanobiotechnol 11(8):965–972
Su H, Liu T, Huang L, Huang J, Cao J, Yang H et al (2018) Plasmonic Janus hybrids for the detection of small metabolites. J Mater Chem B 6(44):7280–7287
Fayed AS, Youssif RM, Salama NN, Elzanfaly ES, Hendawy HA (2021) Utility of silver-nanoparticles for nano spectrofluorimetric determination of meropenem and ertapenem: bio-analytical validation. Spectrochim Acta Part A Mol Biomol Spectrosc 262:120077
Chen Y, Shen C, Wang J, Xiao G, Luo G (2018) Green synthesis of Ag–TiO2 supported on porous glass with enhanced photocatalytic performance for oxidative desulfurization and removal of dyes under visible light. ACS Sustain Chem Eng 6(10):13276–13286
Liao W, Zhang Y, Zhang M, Murugananthan M, Yoshihara S (2013) Photoelectrocatalytic degradation of microcystin-LR using Ag/AgCl/TiO2 nanotube arrays electrode under visible light irradiation. Chem Eng J 231:455
Wang D, Li Y, Li Puma G, Wang C, Wang P, Zhang W et al (2015) Mechanism and experimental study on the photocatalytic performance of Ag/AgCl @ chiral TiO2 nanofibers photocatalyst: the impact of wastewater components. J Hazard Mater 285:277–284
Victora R (1997) Calculated electronic structure of silver halide crystals. Phys Rev B 56(8):4417
Dai YD, Lyu RJ, Wu T, Huang CC, Lin Y-W (2020) Influences of silver halides AgX (X= Cl, Br, and I) on magnesium bismuth oxide photocatalyst in methylene blue degradation under visible light irradiation. J Photochem Photobiol A 397:112585
Fan Y, Han D, Song Z, Sun Z, Dong X, Niu L (2018) Regulations of silver halide nanostructure and composites on photocatalysis. Adv Compos Hybrid Mater 1(2):269–299
Thakur P, Raizada P, Singh P, Kumar A, Khan AAP, Asiri AM (2020) Exploring recent advances in silver halides and graphitic carbon nitride-based photocatalyst for energy and environmental applications. Arab J Chem 13(11):8271–8300
Huang S, Xu Y, Xie M, Ma Y, Yan J, Li Y et al (2018) Multifunctional C-doped CoFe2O4 material as cocatalyst to promote reactive oxygen species generation over magnetic recyclable C-CoFe/Ag–AgX photocatalysts. ACS Sustain Chem Eng 6(9):11968–11978
Liu Y, Zeng X, Hu X, Xia Y, Zhang X (2021) Solar-driven photocatalytic disinfection over 2D semiconductors: the generation and effects of reactive oxygen species. Solar Rrl 5(6):2000594
Ha M, Kim JH, You M, Li Q, Fan C, Nam JM (2019) Multicomponent plasmonic nanoparticles: from heterostructured nanoparticles to colloidal composite nanostructures. Chem Rev 119(24):12208–12278
Hu C, Peng T, Hu X, Nie Y, Zhou X, Qu J et al (2010) Plasmon-induced photodegradation of toxic pollutants with Ag−AgI/Al2O3 under visible-light irradiation. J Am Chem Soc 132(2):857–862
Liu Y, Zhang K, Tian X, Zhou L, Liu J, Liu B (2021) Quantitative single-particle fluorescence imaging elucidates semiconductor shell influence on Ag@TiO2 photocatalysis. ACS Appl Mater Interfaces 13(6):7680–7687
Temerov F, Pham K, Juuti P, Mäkelä JM, Grachova EV, Kumar S et al (2020) Silver-decorated TiO2 inverse opal structure for visible light-induced photocatalytic degradation of organic pollutants and hydrogen evolution. ACS Appl Mater Interfaces 12(37):41200–41210
Wen XJ, Shen CH, Fei ZH, Fang D, Liu ZT, Dai JT et al (2020) Recent developments on AgI based heterojunction photocatalytic systems in photocatalytic application. Chem Eng J 383:123083
Sherry LJ, Chang SH, Schatz GC, Van Duyne RP, Wiley BJ, Xia Y (2005) Localized surface plasmon resonance spectroscopy of single silver nanocubes. Nano Lett 5(10):2034–2038
Sherry LJ, Jin R, Mirkin CA, Schatz GC, Van Duyne RP (2006) Localized surface plasmon resonance spectroscopy of single silver triangular nanoprisms. Nano Lett 6(9):2060–2065
Zhang H, Itoi T, Konishi T, Izumi Y (2019) Dual photocatalytic roles of light: charge separation at the band gap and heat via localized surface plasmon resonance to convert CO2 into CO over silver–zirconium oxide. J Am Chem Soc 141(15):6292–6301
Chen YZ, Li WH, Li L, Wang LN (2018) Progress in organic photocatalysts. Rare Met 37(1):1–12
Fukuzumi S, Ohkubo K (2013) Selective photocatalytic reactions with organic photocatalysts. Chem Sci 4(2):561–574
Liu H, Xu C, Li D, Jiang HL (2018) Photocatalytic hydrogen production coupled with selective benzylamine oxidation over MOF composites. Angew Chem 130(19):5477–5481
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249
Farooq MA, Aquib M, Farooq A, Haleem Khan D, Joelle Maviah MB, Sied Filli M et al (2019) Recent progress in nanotechnology-based novel drug delivery systems in designing of cisplatin for cancer therapy: an overview. Artif Cells Nanomed Biotechnol 47(1):1674–1692
Jahanban-Esfahlan R, Seidi K, Banimohamad-Shotorbani B, Jahanban-Esfahlan A, Yousefi B (2018) Combination of nanotechnology with vascular targeting agents for effective cancer therapy. J Cell Physiol 233(4):2982–2992
Song W, Anselmo AC, Huang L (2019) Nanotechnology intervention of the microbiome for cancer therapy. Nat Nanotechnol 14(12):1093–1103
Zhao CY, Cheng R, Yang Z, Tian ZM (2018) Nanotechnology for cancer therapy based on chemotherapy. Molecules 23(4):826
Fan W, Yung B, Huang P, Chen X (2017) Nanotechnology for multimodal synergistic cancer therapy. Chem Rev 117(22):13566–13638
Misra R, Acharya S, Sahoo SK (2010) Cancer nanotechnology: application of nanotechnology in cancer therapy. Drug Discov Today 15(19–20):842–850
Wang X, Yang L, Chen Z, Shin DM (2008) Application of nanotechnology in cancer therapy and imaging. CA Cancer J Clin 58(2):97–110
Bonnett R (1995) Photosensitizers of the porphyrin and phthalocyanine series for photodynamic therapy. Chem Soc Rev 24(1):19–33
Loschenov V, Konov V, Prokhorov A (2000) Photodynamic therapy and fluorescence diagnostics. Laser Phys Lawrence 10(6):1188–1207
O’Connor AE, Gallagher WM, Byrne AT (2009) Porphyrin and nonporphyrin photosensitizers in oncology: preclinical and clinical advances in photodynamic therapy. Photochem Photobiol 85(5):1053–1074
Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO et al (2011) Photodynamic therapy of cancer: an update. CA Cancer J Clin 61(4):250–281
Berlien HP (2016) Photodynamic therapy (PDT). J Biophotonics 9(11–12):1300–1301
Joseph B, Janam P, Narayanan S, Anil S (2017) Is antimicrobial photodynamic therapy effective as an adjunct to scaling and root planing in patients with chronic periodontitis? A systematic review. Biomolecules 7(4)
Rivas Aiello MB, Castrogiovanni D, Parisi J, Azcarate JC, Garcia Einschlag FS, Gensch T et al (2018) Photodynamic therapy in HeLa cells incubated with riboflavin and pectin-coated silver nanoparticles. Photochem Photobiol 94(6):1159–1166
Wysocka-Król K, Olsztyńska-Janus S, Plesch G, Plecenik A, Podbielska H, Bauer J (2018) Nano-silver modified silica particles in antibacterial photodynamic therapy. Appl Surf Sci 461:260–268
Donohoe C, Senge MO, Arnaut LG, Gomes-da-Silva LC (2019) Cell death in photodynamic therapy: from oxidative stress to anti-tumor immunity. Biochim Biophys Acta Rev Cancer 1872(2):188308
Wang G, Mitomo H, Matsuo Y, Shimamoto N, Niikura K, Ijiro K (2013) DNA-templated plasmonic Ag/AgCl nanostructures for molecular selective photocatalysis and photocatalytic inactivation of cancer cells. J Mater Chem B 1(43):5899–5907
Seo JH, Jeon WI, Dembereldorj U, Lee SY, Joo S-W (2011) Cytotoxicity of serum protein-adsorbed visible-light photocatalytic Ag/AgBr/TiO2 nanoparticles. J Hazard Mater 198:347–355
Kösters JP, Gøtzsche PC (2003) Regular self‐examination or clinical examination for early detection of breast cancer. Cochrane Database Syst Rev (2)
King A, Woo J, Ai Q, Chan J, Lam W, Tse I et al (2019) Complementary roles of MRI and endoscopic examination in the early detection of nasopharyngeal carcinoma. Ann Oncol 30(6):977–982
Wang GL, Xu XF, Qiu L, Dong YM, Li ZJ, Zhang C (2014) Dual responsive enzyme mimicking activity of AgX (X=Cl, Br, I) nanoparticles and its application for cancer cell detection. ACS Appl Mater Interfaces 6(9):6434–6442
Song Y, Qu K, Zhao C, Ren J, Qu X (2010) Graphene oxide: intrinsic peroxidase catalytic activity and its application to glucose detection. Adv Mater 22(19):2206–2210
Tao Y, Lin Y, Huang Z, Ren J, Qu X (2013) Incorporating graphene oxide and gold nanoclusters: a synergistic catalyst with surprisingly high peroxidase-like activity over a broad pH range and its application for cancer cell detection. Adv Mater 25(18):2594–2599
Bharali DJ, Lucey DW, Jayakumar H, Pudavar HE, Prasad PN (2005) Folate-receptor-mediated delivery of InP quantum dots for bioimaging using confocal and two-photon microscopy. J Am Chem Soc 127(32):11364–11371
Song Y, Shi W, Chen W, Li X, Ma H (2012) Fluorescent carbon nanodots conjugated with folic acid for distinguishing folate-receptor-positive cancer cells from normal cells. J Mater Chem 22(25):12568–12573
Zhang B, Wang X, Zhao Y, Lv J, Meng H, Chang H et al (2017) Highly photosensitive colorimetric immunoassay for tumor marker detection based on Cu2+ doped Ag–AgI nanocomposite. Talanta 167:111–117
Mazhabi RM, Ge L, Jiang H, Wang X (2018) A label-free aptamer-based cytosensor for specific cervical cancer HeLa cell recognition through a gC3N4–AgI/ITO photoelectrode. J Mater Chem B 6(31):5039–5049
Cui D, Shi Y, Xing D, Yang S (2021) Ultrahigh sensitive and tumor-specific photoacoustography in NIR-II region: optical writing and redox-responsive graphic fixing by AgBr@ PLGA nanocrystals. Nano Lett 21(16):6914–6922
Mantri Y, Davidi B, Lemaster JE, Hariri A, Jokerst JV (2020) Iodide-doped precious metal nanoparticles: measuring oxidative stress in vivo via photoacoustic imaging. Nanoscale 12(19):10511–10520
Li P, Poon YF, Li W, Zhu H-Y, Yeap SH, Cao Y et al (2011) A polycationic antimicrobial and biocompatible hydrogel with microbe membrane suctioning ability. Nat Mater 10(2):149–156
Zhang L-j, Guerrero-Juarez CF, Hata T, Bapat SP, Ramos R, Plikus MV et al (2015) Dermal adipocytes protect against invasive Staphylococcus aureus skin infection. Science 347(6217):67–71
Zhou S, Li J, Gilroy KD, Tao J, Zhu C, Yang X et al (2016) Facile synthesis of silver nanocubes with sharp corners and edges in an aqueous solution. ACS Nano 10(11):9861–9870
Shah ZH, Wang J, Ge Y, Wang C, Mao W, Zhang S et al (2015) Highly enhanced plasmonic photocatalytic activity of Ag/AgCl/TiO2 by CuO co-catalyst. J Mater Chem A 3(7):3568–3575
Zhu M, Chen P, Liu M (2011) Graphene oxide enwrapped Ag/AgX (X= Br, Cl) nanocomposite as a highly efficient visible-light plasmonic photocatalyst. ACS Nano 5(6):4529–4536
Yang Y, Zhang W, Liu R, Cui J, Deng C (2018) Preparation and photocatalytic properties of visible light driven Ag-AgBr-RGO composite. Sep Purif Technol 190:278–287
Zhang J, Wang J, Xu H, Lv X, Zeng Y, Duan J et al (2019) The effective photocatalysis and antibacterial properties of AgBr/AgVO3 composites under visible-light. RSC Adv 9(63):37109–37118
Lin L, Xie Q, Zhang M, Liu C, Zhang Y, Wang G et al (2020) Construction of Z-scheme Ag–AgBr/BiVO4/graphene aerogel with enhanced photocatalytic degradation and antibacterial activities. Colloids Surf A 601:124978
Li Z, Fu Y, Fang W, Li Y (2015) Electrochemical impedance immunosensor based on self-assembled monolayers for rapid detection of Escherichia coli O157:H7 with signal amplification using lectin. Sensors 15(8):19212–19224
Joung CK, Kim HN, Lim MC, Jeon TJ, Kim HY, Kim YR (2013) A nanoporous membrane-based impedimetric immunosensor for label-free detection of pathogenic bacteria in whole milk. Biosens Bioelectron 44:210–215
Tan F, Leung PHM, Liu ZB, Zhang Y, Xiao L, Ye W et al (2011) A PDMS microfluidic impedance immunosensor for E. coli O157:H7 and Staphylococcus aureus detection via antibody-immobilized nanoporous membrane. Sens Actuators B Chem 159(1):328–335
Varshney M, Li Y, Srinivasan B, Tung S (2007) A label-free, microfluidics and interdigitated array microelectrode-based impedance biosensor in combination with nanoparticles immunoseparation for detection of Escherichia coli O157:H7 in food samples. Sens Actuators B Chem 128(1):99–107
Wu S, Zhang J, Wu P (2019) Photo-modulated nanozymes for biosensing and biomedical applications. Anal Methods 11(40):5081–5088
Liu Y, Wang X, Wei H (2020) Light-responsive nanozymes for biosensing. Analyst 145(13):4388–4397
Hashemi SA, Mousavi SM, Bahrani S, Ramakrishna S (2020) Polythiophene silver bromide nanostructure as ultra-sensitive non-enzymatic electrochemical glucose biosensor. Eur Polym J 138:109959
Chen F, Liang W, Qin X, Jiang L, Zhang Y, Fang S et al (2021) Ag@AgCl photocatalyst loaded on the 3D graphene/PANI hydrogel for the enhanced adsorption-photocatalytic degradation and in situ SERS monitoring properties. ChemistrySelect 6(17):4166–4177
Zhu JH, Feng YG, Wang AJ, Mei LP, Luo X, Feng JJ (2021) A signal-on photoelectrochemical aptasensor for chloramphenicol assay based on 3D self-supporting AgI/Ag/BiOI Z-scheme heterojunction arrays. Biosens Bioelectron 181:113158
Zhang L, Shen YL, Fan GC, Xiong M, Yu XD, Zhao WW (2019) Preparation of an AgI/CuBi2O4 heterojunction on a fluorine-doped tin oxide electrode for cathodic photoelectrochemical assays: application to the detection of L-cysteine. Mikrochim Acta 186(5):284
Yan W, Feng X, Chen X, Hou W, Zhu JJ (2008) A super highly sensitive glucose biosensor based on Au nanoparticles-AgCl@polyaniline hybrid material. Biosens Bioelectron 23(7):925–931
Wu J, Wang X, Wang Q, Lou Z, Li S, Zhu Y et al (2019) Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes (II). Chem Soc Rev 48(4):1004–1076
Li Y, Liu J (2021) Nanozyme’s catching up: activity, specificity, reaction conditions and reaction types. Mater Horiz 8(2):336–350
Zandieh M, Liu J (2021) Nanozyme catalytic turnover and self-limited reactions. ACS Nano 15(10):15645–15655
Ozdemir C, Yeni F, Odaci D, Timur S (2010) Electrochemical glucose biosensing by pyranose oxidase immobilized in gold nanoparticle-polyaniline/AgCl/gelatin nanocomposite matrix. Food Chem 119(1):380–385
Hao N, Zhang X, Zhou Z, Hua R, Zhang Y, Liu Q et al (2017) AgBr nanoparticles/3D nitrogen-doped graphene hydrogel for fabricating all-solid-state luminol-electrochemiluminescence Escherichia coli aptasensors. Biosens Bioelectron 97:377–383
Acknowledgements
We thank all researchers that discussed and helped us with this review.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
LZ contributed to conceptualization, investigation of these related articles and journals, formal analysis, summary, and writing of the original draft. HZ reviewed and edited the final draft and supervised the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors gave their consent for publication.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, L., Zhang, H. Silver Halide-Based Nanomaterials in Biomedical Applications and Biosensing Diagnostics. Nanoscale Res Lett 17, 114 (2022). https://doi.org/10.1186/s11671-022-03752-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11671-022-03752-x