Abstract
BiPO4/Bi2S3 photocatalysts were successfully synthesized by a simple two-step hydrothermal process, which involved the initial formation of BiPO4 rod and then the attachment of Bi2S3 through ion exchange. The as-synthesized products were characterized by X-ray diffraction (XRD), scanning electron microscope (SEM), transmission electron microscopy (TEM), X-ray photoelectron spectroscopy (XPS), and UV-vis diffuse reflectance spectra (UV-vis DRS). It was found that BiPO4 was regular rods with smooth surfaces. However, BiPO4/Bi2S3 heterojunction had a rough surface, which could be attributed to the attachment of Bi2S3 on the surface of BiPO4 rods. The BiPO4/Bi2S3 composite exhibited better photocatalytic performance than that of pure BiPO4 and Bi2S3 for the degradation of methylene blue (MB) and Rhodamine B (RhB) under visible light. The enhanced photocatalytic performance could be ascribed to synergistic effect of BiPO4/Bi2S3 heterojunction, in which the attached Bi2S3 nanoparticles could improve visible-light absorption and the BiPO4/Bi2S3 heterojunction suppressed the recombination of photogenerated electron-hole pairs. Our work suggested that BiPO4/Bi2S3 heterojunction could be a potential photocatalyst under visible light.
Similar content being viewed by others
Background
Currently, semiconductor photocatalysts have attracted a lot of interests due to their widely applications for the degradation of organic contaminants [1–4] and generation of hydrogen from water [5]. Generally speaking, a highly efficient photocatalyst must have a wide photoabsorption range, as well as the low recombination rate of photogenerated electron-hole pairs. Therefore, it is also a challenge to develop a new compound with high photocatalytic efficiency under visible light [6–9].
As a potential photocatalyst, BiPO4 has recently been extensively studied [10–12]. It has been reported that the photocatalytic activity of BiPO4 is strongly dependent on its crystal structure [13] and the monoclinic phase BiPO4 showed a better photocatalytic performance than that of P25 for the photodegradation of organic contaminants under UV irradiation [14]. However, BiPO4 had a wide band gap of about 3.8 eV and thus can only be excited by UV light to generate electron-hole pairs [11]. In order to improve the visible-light utilization of BiPO4, many efforts have been taken. Lin et al. fabricated Ag3PO4/BiPO4 heterojunction with enhanced photocatalytic ability under visible-light irradiation [15]. Duo et al. reported that BiPO4/BiOCl heterojunction also had enhanced photocatalytic activity [16]. Li et al. found that BiPO4/g-C3N4 heterojunction could efficiently respond to visible-light irradiation [17]. Besides, Zhang et al. reported that BiPO4/reduced graphene oxide composites with specific surface areas had better photocatalytic activity for the degradation of MB [18]. Whereas, coupling of BiPO4 with other semiconductors is still meaningful for improving light absorption in the visible spectrum and suppressing the recombination of the photogenerated electron-hole pairs more effectively.
Bi2S3, a small band gap semiconductor (1.3 eV), has a high photoabsorption coefficient [19–21]. Hence, it can usually be used as a potential visible-light photocatalyst through combination from other semiconductors to improve light absorption and separation efficiency of photogenerated electron-hole pairs, such as CdS/Bi2S3 [22], BiVO4/Bi2S3 [23], Bi2S3/BiOBr [24], and so on.
In this study, we reported the preparation of a novel BiPO4/Bi2S3 heterostructure and their photocatalytic properties were evaluated by the degradation of MB and RhB under visible light. As expected, the as-prepared BiPO4/Bi2S3 heterojunction exhibited enhanced visible-light photocatalytic activity and a possible mechanism was presented.
Methods
Materials and Preparation
All reagents were of analytical purity (Sinopharm Chemical reagent Co., Ltd., China) and used without further purification.
Synthesis of BiPO4
BiPO4 was prepared by a facile hydrothermal method. Firstly, 0.5 g of PVP was dissolved in a beaker with deionized water (50 mL) under stirring. Secondly, Bi(NO3)3 · 5H2O and NaH2PO4 · 12H2O (molar radio of 1:1) were added into the solution. After the pH of the reaction system was adjusted to 3 by HNO3, the solution was transferred into a 100-mL Teflon-lined stainless steel autoclave and heated at 180 °C for 24 h. When the system cooled down to room temperature naturally, the resulting product was harvested and washed with deionized water and absolute alcohol for several times. Finally, the as-prepared products were dried at 60 °C for 12 h.
Synthesis of BiPO4/Bi2S3 Photocatalyst
The BiPO4/Bi2S3 photocatalyst was prepared through an in situ ion exchange process. Typically, 0.1 g of PVP was dissolved in 50 mL of ethylene glycol, followed by the addition of 0.456 g of BiPO4 under stirring to achieve suspension. Then, a certain amount of thiourea (the amount of thiourea was 0.086, 0.172, and 0.573 g, and they are named as BB-1, BB-2, and BB-3, respectively.) was added into above suspension and the solution was transferred into a 100-mL Teflon-lined stainless steel autoclave, which was sealed and maintained at 140 °C for 3 h. After the autoclave was cooled to room temperature naturally, the precipitates were collected and washed with water and ethanol several times. The BiPO4/Bi2S3 products were dried at 60 °C for 12 h. For comparison, pure Bi2S3 was prepared through hydrothermal method according to the literature [25].
Characterization of the As-prepared Samples
The phase of the samples was measured by XRD (D/Max-ШC, Shimadzu) using an X-ray diffractometer with Cu Kα radiation. The morphology was analyzed by SEM on Hitachi S-4600 and TEM (FEI Tecnai G20). UV-vis DRS was tested on a Shimadzu UV240 UV-vis spectrophotometer with BaSO4 as a reference material. The elemental composition of the samples was analyzed by X-ray photoelectron spectrometer (XPS, USA Thermo ESCALAB 250).
Photocatalytic Activity
The photocatalytic performance of BiPO4/Bi2S3 heterojunction photocatalyst was evaluated by the degradation of MB and RhB under visible light. In each experiment, 50 mg of different photocatalysts were added into 100 mL of MB or RhB solution (10 mg/L) in a reactor. Before irradiation, the mixture was magnetically stirred for 30 min in the dark to achieve the adsorption/desorption equilibrium between dye and photocatalysts. Then, the solution was irradiated by visible light under continuous stirring. At a defined time interval, about 3 mL of solution was extracted from the reactors and then centrifuged to remove catalysts before analysis. Finally, MB (RhB) solution was analyzed through a UV-vis spectrophotometer. The degradation rate could be obtained through the formula [26]: η = C i /C 0 × 100 %, where C i was the absorbance of MB (RhB) which was measured every 30 min, and C 0 was the absorbance of MB (RhB) before light up.
Results and Discussion
Phase and Crystal Structure Analysis
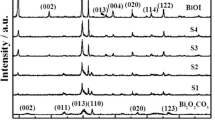
Figure 1 shows the XRD patterns of BiPO4 and BiPO4/Bi2S3 heterojunction with different Bi2S3 contents. In the pure BiPO4, all the diffraction peaks are well matched with the monoclinic phase of BiPO4 (JCPDS File No. 89–0287), indicating that the as-prepared BiPO4 has the high purity. On the other hand, the BiPO4/Bi2S3 composites exhibit a mixture of two crystalline phases. One can be identified as BiPO4, and the others originate from rutile Bi2S3 [25]. Furthermore, the intensities of corresponding to diffraction peaks of Bi2S3 gradually strengthen along with the increase of the Bi2S3 content, while those of BiPO4 simultaneously weaken. No other characteristic peaks of impurity are detected, suggesting that BiPO4/Bi2S3 composites are only composed of BiPO4 and Bi2S3 phases.
The surface chemical composition of BB-2 is analyzed by XPS and the results are shown in Fig. 2. The XPS survey spectrum (Fig. 2a) shows that BB-2 contains Bi, P, S, and O elements, which is consistent to XRD results. Besides, C 1 s peak is also seen in XPS survey spectrum, which can be attributed to adventitious hydrocarbon from instrument. Two peaks appear at 163.97 and 158.65 eV in Fig. 2b, which are corresponding to Bi 4f5/2 and Bi 4f7/2 peaks of Bi3+, respectively [27]. In Fig. 2c, O 1 s peak appeared at 529.59 eV, in which it can be attributed to lattice oxygen in crystalline BiPO4 [28]. In Fig. 2d, the P 2p XPS peak appeared at 131.79 eV, suggesting that P exists in the oxidation of P5+. On the other hand, the binding energies of 164.12 and 158.76 eV are attributed to S 2p peaks (Fig. 2e), which prove the existence of S2− [29].
Morphology Analysis
Figure 3 shows the SEM images of BiPO4 and BiPO4/Bi2S3 composites. It can be seen from Fig. 3a that pure BiPO4 shows regular rod shape with diameter of 200–400 nm and the length of 500–2000 nm. It should be noted that these rods have smooth surfaces. Figure 3b–d shows the SEM images of different BiPO4/Bi2S3 composites. Compared with pure BiPO4, the surfaces of BiPO4/Bi2S3 composites become rough. Furthermore, with the increasing amount of additive thiourea, more Bi2S3 nanoparticles can be observed on the surface of BiPO4 rods gradually, which is also consistent to XRD results.
TEM and HRTEM images are shown in Fig. 4, which display identified results as those of SEM analysis. From Fig. 4a, one can see that pure BiPO4 are regular rods with a smooth surface. While BiPO4/Bi2S3 heterojunction shows a rough surface, suggesting the successful attachment of Bi2S3 on the surface of BiPO4 rods. Furthermore, the lattice spacings can be clearly seen in the corresponding HRTEM image (Fig. 4d). The fringe spacing of 0.47 nm is indexed to the (1 1 0) lattice plane of monoclinic BiPO4, while 0.32 nm is agreed with the (1 0 2) lattice plane of Bi2S3. Therefore, it can be summarized that BiPO4/Bi2S3 heterojunction is achieved through a facile ion-exchange method.
UV-vis Analysis
Figure 5a shows UV-vis DRS of as-prepared BiPO4, Bi2S3, and BiPO4/Bi2S3 composites. It reveals that BiPO4/Bi2S3 composites have a stronger absorption than that of BiPO4 in visible light. The band gap energy can be achieved through the formula [30, 31]. Besides, according to the literature, n values of BiPO4 and Bi2S3 are 4 [32] and 1 [33], respectively. Therefore, as is shown in Fig. 5b, E g of BiPO4 and Bi2S3 can be calculated as 4.08 and 1.30 eV. Moreover, E g of BB-1, BB-2, and BB-3 are 4.01, 3.93, and 3.81 eV, respectively. Besides, Bi2S3 displays quantum size effect, which may influence the band gap, the position of both CB and VB band. Besides, the band gap shift relative to the bulk can be calculated by the following formula [34, 35]:
in which ∆E g (R) is the band gap shift, h is the Planck’s constant, and R is the crystal radius. Besides, m o is electron mass and m e * and m h * are the effective masses of electrons and holes, respectively. Then, the size of Bi2S3 nanoparticles attached on the surface of BiPO4 rods can be calculated as 2.68, 2.72, and 2.78 nm, respectively, which is much smaller than Bohr excitation radius of 24 nm. Therefore, quantum size confinement can be observed obviously, which influences the band gap, the position of both CB and VB band, etc. These results also support the enhancement of photocatalytic activity.
Photocatalytic Activity of Different Samples
The photocatalytic performance of BiPO4/Bi2S3 heterojunction was assessed by photodegradation of MB under visible-light irradiation (Fig. 6a). It can be seen that pure BiPO4 shows poor photocatalytic ability in degrading MB (40 %). Interestingly, the coupling of BiPO4 with Bi2S3 leads to notable enhancement MB photodegradation. The MB removal rates are about 50, 80, and 60 %, respectively. Meantime, RhB here is also employed as an organic pollutant to further confirm the photodegradation activity of BiPO4/Bi2S3 heterojunction. As shown in Fig. 6b, BiPO4/Bi2S3 composites show better photocatalytic activity in the degradation of RhB than that of pure BiPO4 and the best photocatalytic property was achieved for BB-2 sample. The enhanced visible-light-driven activity of the heterostructure must be attributed to the synergistic effect between BiPO4 and Bi2S3. What is more, the quantum size confinement of Bi2S3 in the visible spectrum also leads to the enhancement of photocatalytic activity. However, the excess Bi2S3 content in BiPO4/Bi2S3 composite will cause their photocatalytic performance to decrease (BB-3). It may be attributed to these reasons: one is reduction of active sites due to the excess Bi2S3 nanoparticles on the surface BiPO4 rod [36]. The other is that excessive narrow band gap Bi2S3 may lower the separation efficiency of electron-hole pairs and further inhibit the photocatalytic activity [37].
Possible Photocatalytic Mechanism
The band positions of BiPO4 and Bi2S3 are evaluated based on the equation [38]. Hence, the valence band and conduction band edge potential (E VB and E CB) of BiPO4 and Bi2S3 are 4.39 eV, 0.31 eV and 1.43 eV, 0.13 eV, respectively. Therefore, the possible mechanism is shown in Fig. 7. Bi2S3 nanoparticles absorb the visible light and give rise to electron-hole pairs. The photo-excited electrons in Bi2S3 CB will transfer to BiPO4 rods and holes are left in Bi2S3 VB, which will decrease recombination rate of photogenerated charge carriers. The electrons in BiPO4 CB can rapidly adsorb O2 to form O2 −•, while the holes can interact with the absorbed H2O to achieve hydroxyl radicals. After then, O2 −• and OH• with strong oxidizability can decompose MB (RhB) to generate CO2 and H2O. Moreover, BiPO4/Bi2S3 heterojunction photocatalysts have a stronger and wider absorption in visible light, which is beneficial to photocatalytic activity.
Conclusions
In summary, we have synthesized the BiPO4/Bi2S3 heterojunction with a facile two-step hydrothermal method. Bi2S3 nanoparticles can be in situ formed on the surface of BiPO4 rods through ion exchange. As the quantum size confinement of Bi2S3 in the visible spectrum, it can be used as photosensitizer. When BiPO4 rods are modified with Bi2S3, the separation of electron-hole pairs could be accelerated and the photoabsorption could be promoted as well. These directly led to the enhancement of photocatalytic activity for the degradation of MB (RhB) under visible-light irradiation, and BB-2 sample exhibits the best photocatalytic property. Degradation rate of MB under visible-light irradiation with BB-2 could reach to 80 % in 3 h, double that of pure BiPO4. Besides, degradation rate of RhB could reach to 99.6 % in 3 h, while it only degraded for 8 % by pure BiPO4.
References
Wetchakun N, Chainet S, PhaniChphant S, Wetchakun K (2015) Efficient photocatalytic degradation of methylene blue over BiVO4/TiO2 nanocomposites. Ceram Int 41:5999–6004
Zhou XF, Lu J, Jiang JJ, Li XB, Lu MN, Yuan GT, Wang ZS, Zheng M, Seo HJ (2014) Simple fabrication of N-doped mesoporous TiO2 nanorods with the enhanced visible light photocatalytic activity. Nanoscale Res Lett 9:34
Liu YM, Liu JZ, Lin YL, Zhang YF, Wei Y (2009) Simple fabrication and photocatalytic activity of S-doped TiO2 under lower power LED visible light irradiation. Ceram Int 35:3061–3065
Chen YZ, Zeng DQ, Zhang K, Lu AL, Wang LS, Peng DL (2014) Au-ZnO hybrid nanomultipods and nanopyramids: one-pot reaction synthesis and photocatalytic properties. Nanoscale 6:874–881
Abe R, Sayama K, Sugihara H (2005) Development of new photocatalytic water splitting into H2 and O2 using two different semiconductor photocatalysts and a shuttle redox mediator IO3 −/I−. J Phys Chem B 109:16052–16061
Zou ZG, Ye JH, Sayama K, Arakawa H (2001) Direct-splitting of water under visible light irradiation with an oxide semiconductor photocatalyst. Nature 414:625–627
Xia JX, Yin S, Li HM, Xu H, Xu YG (2011) Improved visible light photocatalytic activity of sphere-like BiOBr hollow and porous structures synthesized via a reactable ionic liquid. Dalton Trans 40:5249–5258
Ismail AA, Bahnemann DW (2011) Mesostructured Pt/TiO2 nanocomposites as highly active photocatalysts for the photooxidation of dichloroacetic acid. J Phys Chem C 115:5784–5791
Fageria P, Gangopadhyay S, Pande S (2014) Synthesis of ZnO/Au and ZnO/Ag nanoparticles and their photocatalytic application using UV and visible light. RSC Adv 4:24962–24972
Zhang YA, Fan HQ, Li MM, Tian HL (2013) Ag/BiPO4 heterostructures: synthesis, characterization and their enhanced photocatalytic properties. Dalton Trans 42:13172–13178
Xu H, Xu YG, Li HM, Xia JX, Xiong J, Yin S, Huang CJ, Wan HL (2012) Synthesis, characterization and photocatalytic property of AgBr/BiPO4 heterojunction. Dalton Trans 41:3387–3394
Lv HW, Shen XP, Ji ZY, Qiu DZ, Zhu GX, Bi YL (2013) Synthesis of graphene oxide-BiPO4 composites with enhanced photocatalytic properties. Appl Surf Sci 284:308–314
Pan CS, Zhu YF (2015) A review of BiPO4, a highly efficient oxyacid-type photocatalyst, used for environmental applications. Catal Sci Technol 5:3071–3083
Pan CS, Zhu YF (2010) New type of BiPO4 oxy-acid salt photocatalyst with high photocatalytic activity on degradation of dye. Environ Sci Technol 44:5570–5574
Lin HL, Ye HF, Xu BY, Cao J, Chen SF (2013) Ag3PO4 quantum dot sensitized BiPO4: A novel p-n junction Ag3PO4/BiPO4 with enhanced visible-light photocatalytic activity. Catal Commun 37:55–59
Duo FF, Wang YW, Mao XM, Zhang XC, Wang YF, Fan CM (2015) A BiPO4/BiOCl heterojunction photocatalyst with enhanced electron-hole separation and excellent photocatalytic performance. Appl Surf Sci 340:35–42
Li ZS, Yang SY, Zhou JM, Li DH, Zhou XF, Ge CY, Fang YP (2014) Novel mesoporous g-C3N4 and BiPO4 nanorods hybrid architectures and their enhanced visible-light-driven photocatalytic performances. Chem Eng J 241:344–351
Zhang YH, Shen B, Huang HW, He Y, Fei B, Lv FZ (2014) BiPO4/reduced graphene oxide composites photocatalyst with high photocatalytic activity. Appl Surf Sci 319:272–277
Chen FJ, Cao YL, Jia DZ (2013) Facile synthesis of Bi2S3 hierarchical nanostructure with enhanced photocatalytic activity. J Colloid Interf Sci 404:110–116
Zhang ZJ, Wang WZ, Wang L, Sun SM (2012) Enhancement of visible-light photocatalysis by coupling with narrow-band-gap semiconductor: a case study on Bi2S3/Bi2WO6. ACS Appl Mater Interf 4:593–597
Manna G, Bose R, Pradhan N (2014) Photocatalytic Au-Bi2S3 heteronanostructures. Angew Chem 126:6861–6864
Fang Z, Liu YF, Fan YT, Ni YH, Wei XW, Tang KB, Shen JM, Chen Y (2011) Epitaxial growth of CdS nanoparticle on Bi2S3 nanowire and photocatalytic application of the heterostructure. J Phys Chem 115:13968–13976
Gao XH, Wu HB, Zheng LX, Zhong YJ, Hu Y, Lou XW (2014) Formation of mesoporous heterostructured BiVO4/Bi2S3 hollow discoids with enhanced photoactivity. Angew Chem 126:6027–6031
Kim J, Kang M (2012) High photocatalytic hydrogen over the band gap-tuned urchin-like Bi2S3-loaded TiO2 composites system. Int J Hydrogen Energy 37:8249–8256
Cui YM, Jia QF, Li HQ, Han JY, Zhu LJ, Li SG, Zou Y, Yang J (2014) Photocatalytic activities of Bi2S3/BiOBr nanocomposites synthesized by a facile hydrothermal process. Appl Surf Sci 290:233–239
Zhou XF, Lu J, Cao JL, Xu MF, Wang ZS (2014) Simple fabrication of rod-like N-doped TiO2/Ag with enhanced visible-light photocatalytic activity. Ceram Int 40:3975–3979
Liu FZ, Shao X, Li HY, Wang M, Yang SR (2013) Facile fabrication of Bi2S3-ZnS nanohybrids on graphene sheets with enhanced electrochemical performances. Mater Lett 108:125–128
Wang KX, Shao CL, Li XH, Zhang X, Lu N, Miao FJ, Liu YC (2015) Hierarchical heterostructures of p-type BiOCl nanosheets in electrospun n-type TiO2 nanofibers with enhanced photocatalytic activity. Catal Commun 67:6–10
Chen DM, Kuang Z, Zhu Q, Du Y, Zhu HL (2015) Synthesis and characterization of CdS/BiPO4 heterojunction photocatalyst. Mater Res Bull 66:262–267
Li L, Zhang XL, Zhang WZ, Wang LL, Chen X, Gao Y (2014) Microwave-assisted synthesis of nanocomposite Ag/ZnO-TiO2 and photocatalytic degradation Rhodamine B with different modes. Colloid Surface A 457:134–141
Fakhri H, Mahjoub AR, Cheshme Khavar AH (2014) Synthesis and characterization of ZnO/CuInS2 nanocomposite and investigation of their photocatalytic properties under visible light irradiation. Appl Surf Sci 318:65–73
Fulekar MH, Singh A, Dutta DP, Roy M, Ballal A, Tyagi AK (2015) Ag incorporated nano BiPO4: sonochemical synthesis, characterization and improved visible light photocatalytic properties. RSC Adv 5:43854–43862
Wang WJ, Cheng HF, Huang BB, Lin XJ, Qin XY, Zhang XY, Dai Y (2013) Synthesis of Bi2O2CO3/Bi2S3 hierarchical microspheres with heterojunctions and their enhanced visible light-driven photocatalytic degradation of dye pollutants. J Colloid Interf Sci 402:34–39
Cheng HF, Huang BB, Qin XY, Zhang XY, Dai Y (2012) A controlled anion exchange strategy to synthesize Bi2S3 nanocrystals/BiOCl hybrid architectures with efficient visible light photoactivity. Chem Commun 48:97–99
Liu XL, Wang WJ, Liu YY, Huang BB, Dai Y, Qin XY, Zhang XY (2015) In situ synthesis of Bi2S3/Bi2SiO5 heterojunction photocatalysts with enhanced visible light photocatalytic activity. RSC Adv 5:55957–55963
Wu ZD, Chen LL, Xing CS, Jiang DL, Xie JM, Chen M (2013) Controlled synthesis of Bi2S3/ZnS microspheres by an in situ ion-exchange process with enhanced visible light photocatalytic activity. Dalton Trans 42:12980–12988
Cao J, Xu BY, Lin HL, Chen SF (2013) Highly improved visible light photocatalytic activity of BiPO4 through fabricating a novel p-n heterojunction BiOI/BiPO4 nanocomposite. Chem Eng J 228:482–488
Ye HF, Lin HL, Cao J, Chen SF, Chen Y (2015) Enhanced visible light photocatalytic activity and mechanism of BiPO4 nanorods modified with AgI nanoparticles. J Mol Catal A Chem 397:85–92
Acknowledgements
The authors are grateful for the financial support of a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions and a key project for Industry-Academia-Research in Jiangsu province (BY2013030-04). This study is also supported by Testing and Analysis Center Soochow University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
The experiments were guided by ML and GY in this work and all the processes were designed by ZW. JG tested and analyzed the dates. YW participated in the discussion and gave useful suggestions. The manuscript was composed by ML. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Lu, M., Yuan, G., Wang, Z. et al. Synthesis of BiPO4/Bi2S3 Heterojunction with Enhanced Photocatalytic Activity under Visible-Light Irradiation. Nanoscale Res Lett 10, 385 (2015). https://doi.org/10.1186/s11671-015-1092-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11671-015-1092-z