Abstract
Through restricting thickness growth by performing coprecipitation at the freezing temperature of ~4 °C, solid-solution nanosheets (up to 5-nm thick) of the Ln2(OH)5NO3·nH2O layered hydroxide (Ln = Y0.98RE0.02; RE = Pr, Sm, Eu, Tb, Dy, Ho, Er, and Tm, respectively) were directly synthesized without performing conventional exfoliation. In situ exchange of the interlayer NO3 − with SO4 2− produced a sulfate derivative [Ln2(OH)5(SO4)0.5·nH2O] of the same layered structure and two-dimensional crystallite morphology but substantially contracted d 002 basal spacing (from ~0.886 to 0.841 nm). The sulfate derivative was systematically compared against its nitrate parent in terms of crystal structure and phase/morphology evolution upon heating. It is shown that the interlayer SO4 2−, owing to its bonding with the hydroxide main layer, significantly raises the decomposition temperature from ~600 to 1000 °C to yield remarkably better dispersed oxide nanopowders via a monoclinic Ln2O2SO4 intermediate. The resultant (Y0.98RE0.02)2O3 nanophosphors were studied for their photoluminescence to show that the emission color, depending on RE3+, spans a wide range in the Commission Internationale de l’Eclairage (CIE) chromaticity diagram, from blue to deep red via green, yellow, orange, and orange red.
Similar content being viewed by others
Background
Y2O3 is a widely used host lattice in the phosphor field, owing to its excellent structure stability, chemical durability, and particularly its ability to accept a substantial amount of various trivalent rare-earth activators for a broad range of optical functionalities. Due to their technical importance in the lighting and display areas, Y2O3-based phosphors are being widely investigated to correlate their luminescent performance with the characteristics of the phosphor powder [1, 2]. Controllable synthesis has always been an active area of phosphor study, and the well-adopted processing technologies may include flux-assisted solid state reaction [3, 4], solution synthesis, combustion [5], spray pyrolysis [6–8], and gas-phase condensation [9, 10].
Among the aforementioned synthetic strategies, solution processing is of particular interest since it allows a facile manipulation of particle morphology. With this technique, Y2O3-based phosphors that have the various morphologies of zero-dimensional (0D) nanoparticles, monodispersed microspheres [11, 12], 1D nanowires/nanotubes [13–15], 2D nanoplates [16–18], and hierarchical structures [2, 19] have been obtained. As the direct product of solution synthesis is usually a precursor, the final phosphor oxide is thus frequently observed to have properties dependent on the characteristics of its precursor [2]. Layered rare-earth hydroxide (LRH), as a relatively new type of anionic layered compounds [20], has attracted much attention during the recent years owing to its unique combination of the layered structure and the abundant optical, magnetic, and catalytic properties of the rare-earth elements [21–34]. The crystal structure of Ln2(OH)5A·nH2O LRH (Ln = rare-earth; A = NO3 − or halogen anion; n ~ 1.5) can be viewed as an alternative stacking along the c-axis ([001] direction) of the positively charged hydroxide main layers containing Ln3+ and exchangeable A anions located in the interlayer for charge balance. The well-established synthetic methodologies of hydrothermal reaction [25–34] and reflux growth [21–24] generally produce platelike LRH crystals of several microns in lateral dimension and tens to hundreds of nanometers in thickness, for which single layer or few-layer thick nanosheets can only be obtained by swelling the pristine crystals via exchange of the interlayer anions with significantly larger ones (such as dodecyl sulfate, DS−), followed by exfoliation in a proper medium (such as formamide) under mechanical agitation [35–39]. Exfoliation, however, is well known to be time consuming, frequently incomplete, and usually accompanied by fragmentation of nanosheets. We previously reported a capped growth technique to synthesize nanometer-thin LRH flakes via one-step hydrothermal reaction [32], but the batch yield is rather limited. Both the pristine LRH crystals and the exfoliated nanosheets can serve as new precursors for oxide phosphors and phosphor films [35–39], but thick crystallites would not collapse into nanoparticles via calcination and the resultant oxides frequently retain platelike morphologies [40–42].
The hydroxide main layer of LRH is a close-packed low-energy plane, and thus, its two-dimensional growth needs lower activation energy than the thickness growth along the [001] direction. We recently demonstrated that, through suppressing thickness growth by lowering the synthesis temperature to ~4 °C, NO3 −-LRH nanosheets of only ~4-nm thick can be directly crystallized, without exfoliation, for a wide spectrum of single Ln (Ln = Pr-Er, and Y) [43]. With this technique, similarly thin nanosheets were produced in this work for the LRH solid solutions of Y/RE (RE = Pr, Sm, Eu, Tb, Dy, Ho, Er, and Tm) in good batch quantity (0.03 mol of LRH or ~10 g). The effects of SO4 2− exchange for interlayer NO3 − on crystal structure and thermal behavior of the nanosheets and also characteristics and luminescent properties of the derived (Y0.98RE0.02)2O3 nanophosphors were studied in detail.
Methods
Freezing Temperature Crystallization of LRH Solid-Solution Nanosheets
The starting rare-earth sources are Pr6O11 (99.96 % pure), Tb4O7 (99.99 % pure), and RE2O3 (99.99 % pure, RE = Y, Sm, Eu, Dy, Ho, Er, and Tm), all were purchased from Huizhou Ruier Rare-Chem. Hi-Tech. Co. Ltd (Huizhou, China). The other reagents of ammonium hydroxide solution (25 %), nitric acid (63 wt.%), and ammonium sulfate are of analytical grade and were purchased from Shenyang Chemical Reagent Factory (Shenyang, China). Nitrate solution of the rare earth was prepared by dissolving the oxide with a proper amount of nitric acid, followed by evaporation to dryness at 95 °C to remove superfluous HNO3 and a final dilution to 1.0 mol/L.
In a typical synthesis, a diluted ammonium hydroxide solution (1.0 mol/L) was slowly dripped (~2.0 mL/min) into 300 mL of a 0.2 mol/L nitrate solution of Ln3+ (Ln = Y0.98RE0.02) kept at ~4 °C until pH ~8 to produce Ln2(OH)5NO3·nH2O nanosheets (30 mmol of Ln2(OH)5NO3·nH2O per batch) [43]. For anion exchange with SO4 2−100 mL aqueous solution containing 15 mmol of (NH4)2SO4 (one SO4 2− would replace two NO3 −) was added into the nanosheets suspension after 20 min of magnetic stirring (in situ anion exchange). The final product was collected via centrifugation after reaction for 1 h, followed by sequential washing with distilled water three times and ethanol one time and then air drying at 50 °C for 15 h. Calcination of the dried nanosheets was performed in flowing oxygen gas (200 mL/min), using a heating rate of 5 °C/min at the ramp stage and a holding time of 4 h. The final phosphor powders are all calcined at 1100 °C, but with the Pr- and Tb-containing samples being subjected to an additional reduction in flowing H2 (200 mL/min) at 1100 °C for 1 h.
Characterization Techniques
Chemical analysis of the products was performed for Ln via inductively coupled plasma (ICP) spectroscopy (Model IRIS Advantage, Jarrell-Ash Japan, Kyoto), for NO3 − via spectrophotometry (Ubest-35, Japan Spectroscopic Co., Ltd., Tokyo), and for S via combustion-infrared absorptiometry (Model CS-444LS, LECO, St. Joseph, MI). The detection limits of these analyses are all 0.01 wt.%. Phase identification was made via X-ray diffractometry (XRD; Model PW3040/60, Panalytical B.V., Almelo, the Netherlands) operated at 40 kV/40 mA, using nickel-filtered Cu-Kα radiation (λ = 0.15406 nm) and a scanning speed of 1.0° 2θ/min. Fourier transform infrared spectroscopy (FTIR; Model Spectrum RXI, Perkin-Elmer, Shelton, CT) was performed by the standard KBr method. Powder morphology was analyzed by transmission electron microscopy under an acceleration voltage of 200 kV (TEM; Model JEM-2000FX, JEOL, Tokyo) and field emission scanning electron microscopy (FE-SEM; Model S-5000, Hitachi, Tokyo) under 10 kV. Thermogravimetry (TG; Model 8120, Rigaku, Tokyo) of the nanosheets was conducted in flowing air (100 mL/min) with a constant heating rate of 10 °C/min. Specific surface area of the oxide phosphor was obtained with an automatic analyzer (Model TriStar II 3020, Micromeritics Instrument Corp., Norcross, GA) using the Brunauer-Emmett-Teller (BET) method via nitrogen adsorption at 77 K. Particle size/size distribution analysis was made with a laser-diffraction particle sizer (Model LA-920, Horiba Scientific, Kyoto), after ultrasonically dispersing the oxide powder in ethanol. Photoluminescence was analyzed at room temperature using an FP-6500 fluorospectrophotometer (JASCO, Tokyo) equipped with a 60-mm-diameter integrating sphere (Model ISF-513, JASCO) and a 150-W Xe-lamp for excitation. Measurements were conducted under identical conditions for all the samples, using a scan speed of 100 nm/min and slit widths of 5 nm for both excitation and emission. Fluorescence lifetime of the luminescence was analyzed with the FP-6500 equipment for Sm3+, Eu3+, and Tb3+, and with a DeltaFlex lifetime fluorescence spectrometer (Horiba Scientific) for the fast decay of Pr3+, Dy3+, Ho3+, Er3+, and Tm3+.
Results and Discussion
Characteristics of the NO3 −-LLnH Nanosheets and the Effects of SO4 2− Exchange
The effects of SO4 2− exchange on the crystal structure of LLnHs were analyzed with L(Y0.98Eu0.02)H for example. Figure 1a compares XRD patterns of the NO3 −-L(Y0.98Eu0.02)H and its SO4 2− derivative. The NO3 −-type exhibits the characteristic 00l and non-00l diffractions of Ln2(OH)5NO3·nH2O layered compounds [20–34]. The strong and sharp 220 diffraction, arising from the ab plane (the hydroxide main layer), suggests that the host layers of L(Y0.98Eu0.02)H are well crystallized. Compared with the thick LRH crystals synthesized under high temperature [21–31, 33, 34], the 002 diffraction has a substantially lower intensity relative to the 220 one, implying that the primary crystallites are much less developed along the c-axis or rather thin, as also confirmed later by TEM analysis. SO4 2− exchange of NO3 − shortens the interlayer distance, as perceived from the obvious shifting of the 00l diffraction to a higher angle, and the basal spacing (d 002) calculated from the center of the 002 peak is ~0.886 nm for NO3 −-L(Y0.98Eu0.02)H and 0.841 nm for the SO4 2− derivative. The values are close to those found for NO3 −-LYH and its exchange product, respectively [43]. The sulfate derivative still exhibits quite strong 220 diffraction, indicating that sulfate exchange did not appreciably damage the hydroxide main layers. Shifting of the 220 peak from 2θ ~ 28.86° to 29.02° and decreased intensity of the 400 diffraction by the anion exchange, however, suggests that the intercalated SO4 2− is interacting with the hydroxide layers to deteriorate crystallinity of the sample. The interaction, mostly through hydrogen bonding with the hydroxyls/H2O in the [Ln(OH)7H2O] and [Ln(OH)8H2O] polyhedrons that comprise the hydroxide layers [21–24], leads to lattice distortion and thus the slight peak-shifting [43]. The hydrogen bonding is also responsible for the observed interlayer contraction, since it would draw closer the adjacent positively charged hydroxide layers [43].
Powder XRD patterns and FT-IR spectra of the products and the 220 spacing (d 220) of the SO4 2−-L(Y0.98RE0.02)H. a Powder XRD patterns and b FT-IR spectra of the NO3 −-L(Y0.98Eu0.02)H and SO4 2−-L(Y0.98Eu0.02)H samples. Part c displays XRD patterns of the rest SO4 2−-L(Y0.98RE0.02)Hs synthesized in this work, and part d is the 220 spacing (d 220) of the SO4 2−-L(Y0.98RE0.02)H, as a function of the ionic radius of the RE3+ dopant (taken for CN = 8)
Chemical analysis of the NO3 −-L(Y0.98Eu0.02)H found ~1.65 wt.% of Eu, 49.88 wt.% of Y, and 16.68 wt.% of NO3 −, corresponding to Y/Eu and NO3 −/Ln (Ln = Y and Eu) molar ratios of 0.98/0.019 and 0.94/2 (close to 1/2), respectively. The results thus confirm that the prescribed Y/Eu atomic ratio (0.98/0.02) has essentially been kept to the product and NO3 −-L(Y0.98Eu0.02)H has been formed. The SO4 2− exchange product was found to have ~1.87 wt.% of Eu, 51.17 wt.% of Y, 4.50 wt.% of S, and trace NO3 −(0.18 wt.%), which lead to Y/Eu and SO4 2−/Ln molar ratios of 0.98/0.021 and 0.48/2 (close to 0.5/2), respectively. The outcomes thus indicate that the anion exchange did not appreciably alter the Y/Eu ratio, and SO4 2− exchange of the interlayer NO3 − is virtually complete. The results of the chemical analysis conform to those of FTIR spectroscopy (Fig. 1b). It is clearly seen that the intense NO3 − absorption at ~1385 cm−1 (ν3 vibration, as of free anion) vanished, and meanwhile the ν3 (~1105 cm−1) and ν1 (~982 cm−1) absorptions, being characteristic of SO4 2−, appeared from the exchange product. The non-splitting feature of ν3 suggests that SO4 2− is not directly coordinated to the metal center in the hydroxide layer while the emergence of ν1 implies that the SO4 2− tetrahedron is distorted owing to the effects of hydrogen bonding [43–45]. It is also owing to the effects of hydrogen bonding that the stretching vibrations of hydroxyls (~3565 cm−1) and the O–H radicals in hydration water (~3370 cm−1) are both substantially enhanced [44, 45]. The twin absorption bands at ~1520 and 1375 cm−1 indicate contamination of the product by CO3 2−, mostly from dissolved atmospheric CO2 during synthesis. All the SO4 2−-LLnHs made in this work show almost identical interlayer distances owing to the limited content of RE, but the 220 diffraction successively shifts towards a higher angle along with the decreasing ionic size of the RE3+ dopant (Fig. 2c). The 220 spacing (d 220) is shown in Fig. 2d as a function of RE3+ size (for eightfold coordination) [46]. As the 220 diffraction reflects metal-to-metal distance in the hydroxide layer [21–24], the d 220 value thus monotonically decreases towards a smaller RE3+ as expected. The results also provide direct evidence of solid-solution formation.
Figure 2 shows the results of electron microscopy for the two types of L(Y0.98Eu0.02)Hs. FE-SEM observation found that the NO3 −-L(Y0.98Eu0.02)H is composed of 3D flower-like assemblies of nanoflakes having lateral dimensions up to ~300 nm (Fig. 2a, coated with 10-nm-thick tungsten for electrical conduction), while TEM analysis found entangled nanosheets of up to ~5-nm thick (the inset). Calculated from the d 002 basal spacing of ~0.886 nm, each single nanosheet would have only ~5–6 stacking repetitions along the c-axis. Selected area electron diffraction (SAED) yielded a well-arranged spot-like pattern (the inset) for the hydroxide layer, indicating that the individual nanosheets are primary of single crystalline and are well crystallized. Anion exchange with SO4 2− did not incur any appreciable morphology change to either the overall flower-like assemblies or the individual nanosheets (Fig. 2b), in compliance with our previous observations on NO3 −-LYH [43].
Decomposition and Phase/Morphology Evolution of the Nanosheets upon Heating
Thermal behaviors of the nanosheets were analyzed via TG, and the results are compared in Fig. 3 for the NO3 −-L(Y0.98Eu0.02)H and SO4 2−-L(Y0.98Eu0.02)H. The NO3 − type clearly decomposes via three well-defined stages as previously observed for thick LRH crystals [25–29, 32–34], with the first one being dehydration to form Ln2(OH)5NO3 (up to ~175 °C), the second one being dehydroxylation to yield an intermediate mass with nominal composition of (Y0.98Eu0.02)2O2(OH)NO3 (up to ~325 °C), and the last one being further dehydroxylation and denitration to form oxide (up to ~545 °C). Though the NO3 −-LLnH obtained in this work are nanometer thin, it shows a thermal behavior almost identical to that reported for thick crystals [25–29, 32–34]. The sulfate derivative similarly decomposes via three steps, but only up to the significantly higher temperature of ~1135 °C and with a rather sluggish step in the ~175–1005 °C range (stage II).
To better understand the thermal decomposition of SO4 2−-L(Y0.98Eu0.02)H, which has not been addressed prior to us, FTIR analysis was performed on the products obtained at various selected temperatures for 4 h (Fig. 4). It is clearly seen that the powders calcined up to 900 °C are characterized by strong SO4 2− absorptions and successively weaker ones of hydroxyls/water. The results may thus imply that the sluggish weight loss observed on the TG curve from ~175 to 1005 °C is mainly owing to successive dehydroxylation rather than desulfuration. The weight loss (~14.4 %) calculated for dehydroxylation of (Y0.98Eu0.02)2(OH)5(SO4)0.5 to the nominal composition of (Y0.98Eu0.02)2O2.5(SO4)0.5 is indeed close to that found via TG (~16.1 %). It is thus plausible to conclude that the slow dehydroxylation of SO4 2−-L(Y0.98Eu0.02)H is mainly due to the intramolecular hydrogen bonding between SO4 2− and OH− groups. Raising the calcination temperature from 900 to 1000 °C simultaneously eliminates the strong SO4 2− and the already rather weak hydroxyl absorptions, suggesting that the sudden weight loss observed on the TG curve from ~1005 to 1135 °C is dominantly resulted from desulfuration. Again, the weight loss calculated for this thermal event (~14.9 %) is close to the value revealed by TG (~13.5 %). The SO4 2− anions exhibit significantly split ν3 and ν4 vibrations for the 800 and 900 °C products, implying that the tetrahedrons of SO4 2− are substantially distorted by direct coordination to Ln3+ [44, 45, 47–49].
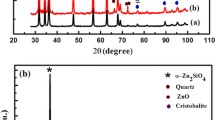
Phase evolution of the SO4 2−-L(Y0.98Eu0.02)H upon heating was studied via XRD analysis of the products calcined at different temperatures, and the results are displayed in Fig. 5. It is seen that dehydration of the layered compound at 200 °C leads to an amorphous mass, which persists up to ~600 °C despite the already occurrence of dehydroxylation (Figs. 3 and 4). Further removal of hydroxyls at 800 °C produced a phase mixture of poorly crystallized cubic Ln2O3 (Ln = Y0.98Eu0.02, JCPDS: 00-043-1036) and monoclinic Ln2O2SO4 (JCPDS: 00-053-0168), whose diffraction intensities both remarkably improve for the 900 °C product. As the original layered compound has the approximate composition of Ln2(OH)5(SO4)0.5·nH2O, it can thus be said that the 900 °C product is approximately composed of 1/2 mol of Ln2O2SO4 and 1/2 mol of Ln2O3. Since the SO4 2− in Ln2O2SO4 is bidentately coordinated to Ln3+ [47–49], the significant splitting of the ν3 and ν4 IR bands was thus observed once the oxy-sulfate compound was formed (Fig. 4). The Ln2O2SO4 component desulfurates at the higher temperature of 1000 °C, and thus, only cubic structured Ln2O3 was found. The results of XRD comply well with those of FTIR (Fig. 4). That is, the ν3 and ν4 vibrations of SO4 2− present as single bands for the 200–600 °C products, as split bands for the 800 and 900 °C products, and vanish for the 1000 °C product. Similar phase evolution analysis of the NO3 −-L(Y0.98Eu0.02)H found that cubic (Y0.98Eu0.02)2O3 crystallizes at ~600 °C via an amorphous state at lower temperatures. The results are not shown here since they are essentially identical to those previously reported by us for thick NO3 −-LRH crystals [33].
XRD patterns of the products calcined from SO4 2−-L(Y0.98Eu0.02)H at the different temperatures. XRD patterns of the products calcined from SO4 2−-L(Y0.98Eu0.02)H at the different temperatures indicated in the figure, where letters C and M denote cubic (Y0.98Eu0.02)2O3 and monoclinic (Y0.98Eu0.02)2O2SO4 phases, respectively
Figure 6 compares XRD patterns of the oxides calcined from SO4 2−-L(Y0.98RE0.02)H at 1100 °C for 4 h. Only enlarged view of the 2θ = 25–35° region was given to show the effects of RE3+ dopant. It is clearly seen that both the (222) and (400) diffractions steadily shift to higher angles along with decreasing ionic radius of RE3+, indicating the formation of solid solution. The lattice parameter (a, in angstrom) calculated from the strongest (222) diffraction indeed becomes successively smaller towards a smaller RE3+ (Fig. 6). The crystallite size assayed from the (222) diffraction by applying Scherrer equation is ~35 nm for all the oxides, irrespective of the dopant type. The cell parameters determined herein are all larger than the 10.547 Å reported for pure Y2O3 (JCPDS: 00-043-1036, Y3+ close to Ho3+ in radius), possibly owing to the limited crystallite size of the present powders.
Morphology evolution of the nanosheets during calcination was studied with NO3 −-L(Y0.98Eu0.02)H and SO4 2−-L(Y0.98Eu0.02)H for example. Figure 7 exhibits typical FE-SEM morphologies for the powders calcined at some representative temperatures. It is seen that the 800 °C product from NO3 −-L(Y0.98Eu0.02)H well retained the overall morphology of its precursor, despite that it has been a phase-pure oxide, and the flower-like assemblies (domains) and the individual nanosheets within the domains are clearly observable. Calcination at 900 °C led to substantial collapse of the nanosheets into nanoparticles within each domain, owing to the thermal stress arising from crystallite growth, and the domain boundary is still identifiable. Significant crystallite growth was observed at 1100 °C, together with densification of some of the domains via inter-particle sintering to form dense aggregates. The final powder was found to have a specific surface area of ~8.3 m2/g, corresponding to an average particle size of ~140 nm. The value is significantly larger than that observed from the FE-SEM micrograph (up to ~90 nm) for the primary particles, due to the presence of hard aggregates. The 800 and 900 °C powders from SO4 2−-L(Y0.98Eu0.02)H show morphologies similar to their counterparts described above, except that the domains in the 900 °C product are much less disintegrated since the Ln2O2SO4 phase (Fig. 5), finely mixed with the Ln2O3 portion, significantly restricts crystallite growth. Calcination to 1100 °C causes complete collapse of the domains to yield a substantially better dispersed oxide powder, and the evolution of SO x gas was believed to promote disintegration of the domains. Accordingly, the final oxide has a much higher specific surface area of ~17.5 m2/g (average particle size ~67 nm). The amount of residual sulfur in the oxide phosphors calcined at 1100 °C was assayed via ICP elemental analysis to be up to 0.18 wt.% in our previous work [50].
Figure 8 shows the particle size/size distribution of the two kinds of (Y0.98Eu0.02)2O3 powders calcined at 1100 °C. It is clearly seen that the powder from NO3 −-L(Y0.98Eu0.02)H exhibits a bimodal size distribution owing to the presence of hard aggregates (Fig. 7). The finer portion (~56.5 vol.%) has an average particle (cluster) size of ~320 ± 43 nm while the coarser part has a value of ~6.21 ± 0.53 μm. A unimodal size distribution was observed for the (Y0.98Eu0.02)2O3 powder from SO4 2−-L(Y0.98Eu0.02)H, and the average particle size was analyzed to be ~219 ± 94 nm. The above results are in agreement with morphology observations (Fig. 7), and further confirm that SO4 2− exchange of the interlayer NO3 − is beneficial to the derivation of finer and better dispersed oxide powders.
Particle size/size distribution analysis of the (Y0.98Eu0.02)2O3 powders. Particle size/size distribution analysis of the (Y0.98Eu0.02)2O3 powders calcined at 1100 °C for 4 h from a the NO3 −-L(Y0.98Eu0.02)H and b the SO4 2−-L(Y0.98Eu0.02)H. The size distribution is given in cumulative volume (right axis)
Photoluminescent Properties of the (Y0.98RE0.02)2O3 Nanophosphors
Figure 9 shows photoluminescence excitation/emission spectra for the (Y0.98RE0.02)2O3 nanophosphors calcined at 1100 °C, with the excitation and emission wavelengths used for the measurements indicated in each part of the figure. The origins of these main bands [19, 51–53] are summarized in Table 1, together with the chromaticity coordinates of emission and the fluorescence lifetime. The origins of the other PLE/PL bands in each part of Fig. 9 are well documented and can be found in the literature [19, 51–53]. It is seen from the Commission Internationale de l’Eclairage (CIE) chromaticity diagram that the phosphors synthesized in this work span a wide range of emission colors, from blue (Tm3+) to deep red (Pr3+) via green (Tb3+, Ho3+, and Er3+), yellow (Dy3+), orange (Sm3+), and orange red (Eu3+).
Conclusions
It is shown in this work that coprecipitation at the freezing temperature of ~4 °C can directly produce, without exfoliation, solid-solution nanosheets of the nitrate-type layered hydroxides of Ln2(OH)5NO3·nH2O (NO3-LLnH, Ln = Y0.98RE0.02, and RE = Pr, Sm, Eu, Tb, Dy, Ho, Er, and Tm). Replacement of the interlayer NO3 − with SO4 2− via in situ anion exchange was achieved to produce the sulfate derivative of SO4 2−-LLnH. Detailed characterizations of both the types of layered materials and their calcination products via the combined techniques of XRD, FTIR, DTA/TG, FE-SEM/TEM, BET, particle sizing, and photoluminescence spectroscopy have led to the following main conclusions: (1) anion exchange did not bring about any appreciable change to the layered structure and the two-dimensional crystallite morphology, but induces a basal-spacing contraction from ~0.886 to 0.841 nm, (2) the interlayer SO4 2− significantly raises the decomposition temperature of the nanosheets from ~600 to 1000 °C to yield oxide via a monoclinic-structured Ln2O2SO4 intermediate phase, and (3) the (Y0.98RE0.02)2O3 powders from SO4 2−-LLnH are much better dispersed and finer than those from NO3-LLnH, and exhibit emission colors, depending on RE3+, covering a wide range in the CIE chromaticity diagram, from blue to deep red via green, yellow, orange, and orange red.
References
Igarashi T, Ihara M, Kusunoki T, Ohno K (2000) Relationship between optical properties and crystallinity of nanometer Y2O3:Eu3+ phosphor. Appl Phys Lett 76:1549–1551
Zhu Q, Li JG, Li XD, Sun XD (2009) Morphology-dependent crystallization and luminescence behavior of (Y, Eu)2O3 red phosphors. Acta Mater 57:5975–5985
Blasse G, Grabmair BC (1994) Luminescent materials. Springer Verlag, Berlin
Hong GY, Jeon BS, Yoo YK, Yoo JS (2001) Photoluminescence characteristics of spherical Y2O3:Eu3+ phosphors by aerosol pyrolysis. J Electrochem Soc 148:H161–H166
Ekambaram S, Patil KC, Mazza M (2005) Synthesis of lamp phosphors: facile combustion approach. J Alloys Compd 393:81–92
Kim EJ, Kang YC, Park HD, Ryu SK (2003) UV and VUV characteristics of (YGd)2O3:Eu phosphor particles prepared by spray pyrolysis from polymeric precursors. Mater Res Bull 38:515–524
Lenggoro IW, Itoh Y, Okuyama K, Kim TO (2004) Nanoparticles of a doped oxide phosphor prepared by direct-spray pyrolysis. J Mater Res 19:3534–3539
Wakefield G, Holland E, Dobson PJ, Hutchison JL (2001) Luminescence properties of nanocrystalline Y2O3:Eu3+. Adv Mater 13:1557–1560
Camenzind A, Strobel R, Pratsinis SE (2005) Cubic or monoclinic Y2O3:Eu3+ nanoparticles by one step flame spray pyrolysis. Chem Phys Lett 415:193–197
Li J-G, Ishigaki T (2012) One-step Ar/O2 thermal plasma processing of Y2O3:Eu3+ red phosphors: phase structure, photoluminescent properties, and the effects of Sc3+ codoping. J Solid State Chem 196:58–62
Li J-G, Li XD, Sun XD, Ishigaki T (2008) Monodispersed colloidal spheres for uniform Y2O3:Eu3+ red-phosphor particles and greatly enhanced luminescence by simultaneous Gd3+ doping. J Phys Chem C 112:11707–11716
Zhu Q, Li J-G, Li XD, Sun XD, Sakka Y (2011) Monodisperse colloidal spheres for (Y, Eu)2O3 red-emitting phosphors: establishment of processing window and size-dependent luminescence behavior. Sci Technol Adv Mater 12:055001
Wu CF, Qin WP, Qin GS (2003) Photoluminescence from surfactant-assembled Y2O3:Eu3+ nanotubes. Appl Phys Lett 82:520–522
Zhang JL, Hong GY (2004) Synthesis and photoluminescence of the Y2O3:Eu3+ phosphor nanowires in AAO template. J Solid State Chem 177:1292–1296
Wan JX, Wang ZH, Chen XY, Mu LY, Qian YT (2005) Shape-tailored photoluminescent intensity of red phosphor Y2O3:Eu3+. J Cryst Growth 284:538–543
He Y, Tian Y, Zhu ZF (2003) Large-scale synthesis of luminescent Y2O3:Eu nanobelts. Chem Lett 32:862–863
Wu X, Tao Y, Gao F, Dong L, Hu Z (2005) Preparation and photoluminescence of yttrium hydroxide and yttrium oxide doped with europium nanowires. J Cryst Growth 277:643–649
Lu ZG, Qian DY, Tang G (2005) Facile synthesis and characterization of sheet-like Y2O3: Eu3+ microcrystals. J Cryst Growth 276:513–518
Lin J, Yu M, Lin CK, Liu XM (2007) Multiform oxide optical materials via the versatile Pechini-type sol-gel process: synthesis and characteristics. J Phys Chem C 111:5835–5845
Gándara F, Perles J, Snejko N, Iglesias M, Gómez-Lor B, Gutiérrez-Puebla E et al (2006) Layered rare-earth hydroxides: a class of pillary crystalline compounds for intercalation chemistry. Angew Chem Int Ed 45:7998–8001
Geng FX, Matsushita Y, Ma R, Xin H, Tanaka M, Izumi F, Iyi N, Sasaki T (2008) General synthesis and structural evolution of a layered family of Ln8(OH)20Cl4·nH2O (Ln = Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, and Y). J Am Chem Soc 130:16344–16350
Geng FX, Xin H, Matsushita Y et al (2008) New layered rare-earth hydroxides with anion-exchange properties. Chem Eur J 14:9255–9260
Geng FX, Matsushita Y, Ma R et al (2008) General synthesis and structural evolution of a layered family of Ln8(OH)20Cl4·nH2O (Ln = Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, and Y). Inorg Chem 130:16344–16350
Geng FX, Ma R, Sasaki T (2010) Anion-exchangeable layered materials based on rare-earth phosphors: unique comination of rare-earth host and exchangeable anions. Acc Chem Res 43:1177–1185
McIntyre LJ, Jackson LK, Fogg AM (2008) Synthesis and anion exchange chemistry of new intercalation hosts containing lanthanide cations, Ln2(OH)5NO3·nH2O (Ln = Y, Gd-Lu). J Phys Chem Solids 69:1070–1074
Poudret L, Prior TJ, McIntyre LJ et al (2008) Synthesis and crystal structures of new lanthanide hydroxyhalide anion exchange materials, Ln2(OH)5X·1.5H2O (X = Cl, Br; Ln = Y, Dy, Er, Yb). Chem Mater 20:7447–7453
McIntyre LJ, Jackson LK, Fogg AM (2008) Ln2(OH)5NO3·xH2O (Ln = Y, Gd-Lu): a novel family of anion exchange intercalation hosts. Chem Mater 20:335–340
Hindocha SA, McIntyre LJ, Fogg AM (2009) Precipitation synthesis of lanthanide hydroxyl nitrate anion exchange materials, Ln2(OH)5NO3·H2O (Ln = Y, Eu-Er). J Solid State Chem 182:1070–1074
McIntyre LJ, Prior TJ, Fogg AM (2010) Observation and isolation of layered and framework ytterbium hydroxide phases using in situ energy-dispersive X-ray diffraction. Chem Mater 22:2635–2645
Lee K-H, Byeon S-H (2009) Extended menberd of the layered rare-earth hydroxides family, RE2(OH)5NO3·nH2O (RE = Sm, Eu, and Gd): synthesis and anion-exchange behavior. Eur J Inorg Chem 7:929–936
Lee K-H, Byeon S-H (2009) Synthesis and aqueous colloidal solutions of RE2(OH)5NO3·nH2O (RE = Nd and La). Eur J Inorg Chem 31:4727–4732
Zhu Q, Li J-G, Zhi CY, Ma RZ, Sasaki T, Xu JX, Liu CH, Li XD, Sun XD, Sakka Y (2011) Nanometer-thin layered hydroxide platelets of (Y0.95Eu0.05)2(OH)5NO3·xH2O: exfoliation-free synthesis, self-assembly, and the derivation of dense oriented oxide films of high transparency and greatly enhanced luminescence. J Mater Chem 21:6903–6908
Zhu Q, Li J-G, Zhi CY, Li XD, Sun XD, Sakka Y, Golberg D, Bando Y (2010) Layered rare-earth hydroxides (LRHs) of (Y1-x Eu x )2(OH)5NO3·nH2O(x = 0-1): structural variations by Eu3+ doping, phase conversion to oxides, and the correlation of photoluminescence behaviors. Chem Mater 22:4204–4213
Zhu Q, Li J-G, Ma RZ, Sasaki T, Yang XJ, Li XD, Sun XD, Sakka Y (2012) Well-defined crystallites autoclaved from the nitrate/NH4OH reaction system as the precursor for (Y, Eu)2O3 red phosphor: crystallization mechanism, phase and morphology control, and luminescent property. J Solid State Chem 192:229–237
Hu LF, Ma RZ, Ozawa TC, Sasaki T (2010) Exfoliation of layered europium hydroxide into unilamellar nanosheets. Chem Asian J 5:248–251
Lee KH, Lee BI, You JH, Byeon SH (2010) Transparent Gd2O3:Eu phosphor layer derived exfoliated layered gadolinium hydroxide nanosheets. Chem Commun 46:1461–1463
Yoon YS, Byeon SH, Lee IS (2010) Unexplored thermal transformation behavior of two- dimensionally bound gadolinium hydroxide layers: fabrication of oriented crystalline films of gadolinium oxychloride nanosheets suitable for the multicolor luminescence with color tenability. Adv Mater 22:3272–3276
Lee BI, Lee ES, Byeon SH (2012) Assembly of layered rare-earth hydroxide nanosheets and SiO2 nanoparticles to fabricate multifunctional transparent films capable of combinatorial color generation. Adv Funct Mater 22:3562–3569
Zhu Q, Li JG, Li XD, Sun XD, Qi Y, Zhu MY et al (2014) Tens of micron sized unilamellar nanosheets of Y/Eu layered rare-earth hydroxide (LRH): efficient exfoliation via fast anion exchange and their self-assembly into oriented oxide film with enhanced photoluminescence. Sci Technol Adv Mater 15:014203
Wu XL, Li J-G, Zhu Q, Li JK, Ma R, Sasaki T, Li XD, Sun XD, Sakka Y (2012) The effects of Gd3+ substitution on the crystal structure, site symmetry, and photoluminescence of Y/Eu layered rare-earth hydroxide (LRH) nanoplates. Dalton Trans 41:1854–1861
Wu XL, Li J-G, Li JK, Zhu Q, Li XD, Sun XD et al (2013) Layered rare-earth hydroxide (LRH) and oxide nanoplates of the Y/Tb/Eu system: phase controlled processing, structure characterization, and color-tunable photoluminescence via selective excitation and efficient energy transfer. Sci Technol Adv Mater 14:015006
Wu XL, Li J-G, Ping D-H, Li JK, Zhu Q, Li XD, Sun XD, Sakka Y (2013) Structure characterization and photoluminescence properties of (Y0.95-x Gd x Eu0.05)2O3 red phosphors converted from layered rare-earth hydroxide (LRH) nanoflake precursors. J Alloys Compd 559:188–195
Wu XL, Li J-G, Zhu Q, Liu WG, Li J, Li XD, Sun XD, Sakka Y (2015) One-step freezing temperature crystallization of layered rare-earth hydroxide (Ln2(OH)5NO3·nH2O) nanosheets for a wide spectrum of Ln (Ln = Pr-Er, and Y), anion exchange with fluorine and sulfate, and microscopic coordination probed via photoluminescence. J Mater Chem C 3:3428–3437
Nakamoto K (1963) Infrared spectra of inorganic and coordination compounds. John Wiley & Sons, New York
Gadsden JA (1975) Infrared spectra of minerals and related inorganic compounds. Butter worth, Newton
Shannon RD (1976) Revised effective ionic-radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr Sect A Cryst Phys Diffr Theor Gen Crystallogr A32:751–767
Liang JB, Ma R, Geng FX, Ebina Y, Sasaki T (2010) Ln2(OH)4SO4·nH2O (Ln = Pr to Tb; n~2): a new family of layered rare-earth hydroxides rigidly pillared by sulfate ions. Chem Mater 22:6001–6007
Wang XJ, Li J-G, Zhu Q, Li XD, Sun XD, Sakka Y (2014) Facile and green synthesis of (La0.95Eu0.05)2O2S red phosphors with sulfate-ion pillared layered hydroxides as a new type of precursor: controlled hydrothermal processing, phase evolution and photoluminescence. Sci Technol Adv Mater 15:014204
Wang XJ, Li J-G, Zhu Q, Li XD, Sun XD, Sakka Y (2014) Synthesis, characterization, and photoluminescent properties of (La0.95Eu0.05)2O2SO4 red phosphors with layered hydroxyl sulfate as precursor. J Alloys Compd 603:28–34
Lu B, Li J-G, Suzuki TS, Estili M, Liu WG, Sun XD, Sakka Y (2015) Controlled synthesis of layered rare-earth hydroxide nanosheets leading to highly transparent (Y0.95Eu0.05)2O3 ceramics. J Am Ceram Soc 98(5):1413–1422
Li J-G, Sakka Y (2015) Recent progress in advanced optical materials based on gadolinium aluminate garnet (Gd3Al5O12). Sci Technol Adv Mater 16:014902
Yang J, Li C, Quan Z, Zhang C, Yang P, Li Y, Yu C, Lin J (2008) Self-assembled 3D flowerlike Lu2O3 and Lu2O3:Ln3+ (Ln = Eu, Tb, Dy, Pr, Sm, Er, Ho, Tm) microarchitectures: ethylene glycol-mediated hydrothermal synthesis and luminescent properties. J Phys Chem C 112:12777–12785
Aumuller GC, Kostler W, Grabmaier BC, Grey R (1994) Luminescence properties of Pr3+ in cubic rare-earth-oxides. J Phys Chem Solids 55:767–772
Acknowledgements
The work is supported in part by the National Natural Science Foundation of China (Grants No. 51172038, 51302032, and 51402059), the Fundamental Research Funds for the Central Universities (Grants N140204002 and N130810003) and Grants-in-Aid for Scientific Research (KAKENHI, No.26420686).
Authors’ contributions
JGL conceived the project and drafted the manuscript. XLW and WGL carried out the experiments. QZ, XDL and XDS were involved in sample characterization and results discussion. All the authors have read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wu, X., Liu, W., Li, JG. et al. Sulfate Exchange of the Nitrate-Type Layered Hydroxide Nanosheets of Ln2(OH)5NO3·nH2O for Better Dispersed and Multi-color Luminescent Ln2O3 Nanophosphors (Ln = Y0.98RE0.02, RE = Pr, Sm, Eu, Tb, Dy, Ho, Er, and Tm). Nanoscale Res Lett 11, 328 (2016). https://doi.org/10.1186/s11671-016-1544-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11671-016-1544-0