Abstract
In present work, two-dimensional g-C3N4 was used to modify TiO2 nanorod array photoanodes for CdS quantum dot-sensitized solar cells (QDSSCs), and the improved cell performances were reported. Single crystal TiO2 nanorods are prepared by hydrothermal method on transparent conductive glass and spin-coated with g-C3N4. CdS quantum dots were deposited on the g-C3N4 modified TiO2 photoanodes via successive ionic layer adsorption and reaction method. Compared with pure TiO2 nanorod array photoanodes, the g-C3N4 modified photoanodes showed an obvious improvement in cell performances, and a champion efficiency of 2.31 % with open circuit voltage of 0.66 V, short circuit current density of 7.13 mA/cm2, and fill factor (FF) of 0.49 was achieved, giving 23 % enhancement in cell efficiency. The improved performances were due to the matching conduction bands and valence bands of g-C3N4 and TiO2, which greatly enhanced the separation and transfer of the photogenerated electrons and holes and effectively suppressed interfacial recombination. Present work provides a new direction for improving performance of QDSSCs.
Similar content being viewed by others
Background
As one kind of novel solar cells, quantum dot-sensitized solar cells (QDSSCs) have attracted worldwide scientific and technological interest [1]. Basically, the structure of a QDSSC includes photoanode (a layer of porous oxide semiconductor with wide bandgap covered by semiconductor QDs as sensitizers), liquid electrolyte, and counter electrode. Many factors such as morphologies of oxide semiconductors, selection of sensitizers, and counter electrodes et al. could greatly affect the photoelectric conversion efficiency (PCE) of QDSSCs. Therefore, many efforts have been devoted to investigate these factors. Recently, a PCE of 9.01 % was achieved using CdSe0.65Te0.35 quantum dot (QD) as sensitizers [2]. However, the PCE of QDSSC is still far behind its theoretical efficiency, and further researches from different aspects are still required to improve the efficiencies of QDSSCs.
TiO2 is one of the most important semiconductors as the photoanode material which is the key components in the configuration of QDSSCs. Since the breakthrough work on colloidal TiO2 based DSSCs by O’Regan and Grätzel in 1991, various TiO2 nanostructures have been used in QDSSC, like nanoparticles, nanosheet, and nanorod [3–8]. Among them, single-crystalline TiO2 nanorod array would be one of the most desirable nanostructures for preparing photoanode of QDSSC due to its effective charge transfer property as well as excellent light harvesting ability. Inorganic semiconductors QDs such as CdS, PbS, PbSe, CdTe, CdSe, and Bi2S3 have been used to assist as a sensitizer for solar devices [9]. Among them, CdS is considered to be one of the potential photovoltaic semiconductive materials for its broadly tunable bandgap. The combination of wide bandgap semiconductors and CdS QDs can preferably collect the visible light used in photoelectrochemical applications.
One of obstacles which limit the performance of QDSSCs is the photogenerated carrier recombination. In order to restrain such recombination, introducing a passivation layer such as Al2O3 and ZnS between photoanode and electrolyte would be an effective method [10], which can retard the recombination by partially separating the electrons and electrolyte. Recently, graphitic carbon nitride (g-C3N4) has drawn much attention as a metal-free photocatalyst due to high photocatalytic efficiency.[11–13] Due to the band structure of g-C3N4, type II band alignment could be formed between g-C3N4 and TiO2, which can significantly prevent the migration of photogenerated electrons from TiO2 and QDs to the electrolyte [14]. Moreover, introducing g-C3N4 could expand the absorption range of sunlight. Therefore, introducing g-C3N4 into TiO2-based photoanodes should improve the performance of QDSSCs.
However, most reports about applications of g-C3N4 are for photocatalysts, and few works for solar cells could be found. Very recently, Wu et al. reported the improved short circuit current of ZnO-based dye-sensitized solar cells (DSSCs) using g-C3N4 as multifunctional protecting layer of ZnO particles [15]. Xu et al. reported enhanced PCE of DSSCs using g-C3N4 modified TiO2 nanosheets [16]. In present work, we investigated the effect of g-C3N4 as both recombination retarding layer and sensitizer on the performance of QDSSC. Single crystal TiO2 nanorod array was prepared by hydrothermal method on a transparent conducting glass and spin-coated with g-C3N4, leading to the formation of g-C3N4/TiO2 heterostructure. Compared with pure TiO2 nanorod array photoanodes, the g-C3N4 modified photoanodes showed an obvious improvement in cell performances. The results of I-V characteristic exhibited that introducing g-C3N4 increased both the open circuit voltage and short circuit photocurrent density, and the possible mechanism is discussed.
Methods
Materials
FTO glasses were purchased from Zhuhai Kaivo Optoelectronic Technology Co., Ltd. Acetone, ethanol, hydrochloric acid, and cadmium acetate and methanol were purchased from Beijing Chemical Works. Titanium butoxide was purchased from Shanghai Chemicals. Melamine, Na2S, S, urea, and acetic acid were purchased from Aladdin. CuSO4 was acquired from Tianjin Guangfu Technology Development Co., Ltd. Na2S2O3 was purchased from Damao Chemical Reagent, Tianjin.
Preparation of TiO2 Nanorod Arrays
TiO2 nanorod array was fabricated according to the previous report [17]. Typically, 15 mL of deionized water was mixed with 15 mL hydrochloric acid. The mixture was then stirred for 15 min followed by the addition of 0.5 mL of titanium butoxide. The mixture was transferred into a 45-mL autoclave. Then, cleaned FTO substrates were put into the autoclave, and the hydrothermal process was conducted at 150 °C for 12 h.
Preparation of g-C3N4 Paste
The g-C3N4 was prepared using the method reported previously [18–20]. Briefly, 3 g melamine and 4 g urea were mixed in a 20-mL crucible, transferred into a muffle furnace, and heated to and kept at 550 °C for 2 h. The yellow crystalline g-C3N4 bulk was obtained and then fully grinded into pale yellow powders. The g-C3N4 paste was prepared by mixing g-C3N4 powders (0.8 g), ethyl cellulose (0.4 g), and α-terpinol (3.245 g) in anhydrous ethanol (8.5 mL) and stirring the mixture for 24 h.
Preparation of CdS/g-C3N4/TiO2 Photoanodes
The g-C3N4 paste was spin-coated on the as-prepared TiO2 nanorod. The as-received g-C3N4/TiO2 nanorod photoanodes were subjected to a sintering process in air at 450 °C for 30 min. After cooling to room temperature, the photoanodes were decorated with CdS QDs by successive ionic layer adsorption and reaction (SILAR) method [21]. The g-C3N4/TiO2 nanorod photoanode was successively dipped in a 0.05 M cadmium acetate methanol solution and a 0.05 M Na2S methanol solution each for 30 s. The two-step dipping procedure was termed as one cycle. The illustration of photoanode structure is shown in Scheme 1.
Preparation of CuS Counter Electrodes
CuS counter electrodes were made by chemical bath deposition (CBD) method. One molar CuSO4 aqueous solution and 1 M Na2S2O3 aqueous solution were mixed with the volume ratio of 1:4. The pH of the mixed solution was adjusted to 2 with acetic acid. Then, the FTO glasses were immersed into 100 mL as-prepared mixed solution. The above solution was heated to 70 °C and kept for 4 h. After cooling down to the room temperature, the substrates were washed and dried in air and then heated to 130 °C and kept for 30 min.
Fabrication of QDSSCs
The as-prepared CdS/g-C3N4/TiO2 nanorod photoanode and CuS counter electrode were assembled to a sandwich-type cell and penetrated with a polysulde electrolyte that consisted of 1 M Na2S and 1 M S in methanol and H2O solution (v/v = 7:3).
Characterization
The samples were characterized using field emission scanning electron microscopy (FESEM, S4800, Hitachi), transmission electron microscopy (TEM) (Tecnai F20), X-ray diffraction (XRD) (D-MAX II A X-ray diffractmeter), X-ray photoelectron spectra (XPS) (VG ESCALAB MKII), and Fourier transform infrared spectroscopy (FTIR) (VERTEX 70). The cell performances were investigated by AM 1.5 solar simulator and Solar Cell Scan 100 (Zolix, Beijing).
Results and Discussion
The morphologies of the as-prepared TiO2 nanorods and g-C3N4/TiO2 nanorods on FTO substrate are shown in Fig. 1. As shown in Fig. 1a, TiO2 nanorods with high density in the average diameter ~100 nm are formed uniformly on FTO substrate. For these nanorods, while the side facets are smooth, the shape of top facets is square and composed of many step edges. These steps are responsible for further growth of the TiO2 nanorod, and these results show the expected growth habit of the tetragonal crystal. From the cross-sectional image of the sample as shown in Fig. 1c, it is obvious that the well-aligned nanorods are nearly normal to the FTO substrate. The length of the nanorods is about 3 μm. For TiO2 nanorods capped by g-C3N4, Fig. 1b shows that discontinuous g-C3N4 layer was coated on the surface of TiO2 nanorods. These remaining vacancies could assure that CdS quantum dots can be deposited on g-C3N4 as well as TiO2 nanorods. The cross-sectional view in Fig. 1d indicates that g-C3N4 was successfully coated on the TiO2 nanorods with the thickness of about 0.8 μm. Figure 1e shows the TEM image of TiO2 nanorod decorated with CdS QDs for 10 cycles. Compared with bare TiO2 nanorod, the rough surface could be observed after CdS QD deposition, indicating that large amounts of CdS QDs had been deposited on the TiO2 nanorods. This is further confirmed by HRTEM image (Fig. 1f). The lattice fringe space of 0.319 and 0.336 nm corresponds to the (110) plane of tetragonal rutile TiO2, and (111) planes of the cubic phase of CdS could be confirmed.
Morphologies of TiO2/FTO and g-C3N4/TiO2/FTO photoelectrodes: typical top view SEM images of TiO2/FTO photoelectrode (a) and g-C3N4/TiO2/FTO photoelectrode (b); typical cross-sectional view of the well-aligned TiO2 nanorod array (c) and g-C3N4/TiO2 photoelectrode (d); typical TEM image of single TiO2 nanorod deposited with CdS QDs (10 cycles) (e), and HRTEM of CdS QD decorated TiO2 nanorod (f)
Figure 2 shows the XRD curves of FTO substrate, TiO2/FTO, g-C3N4/TiO2/FTO, and CdS/g-C3N4/TiO2/FTO, respectively. The XRD result of TiO2/FTO exhibits a greatly increased (002) and (101) diffraction, suggesting the vertical growth of highly oriented titania nanorods on FTO, which is consistent with SEM observation. After coating with g-C3N4, a peak at 27.7° could be observed which is attributed to the typical (002) plane of the g-C3N4. After the deposition of CdS QDs, the XRD pattern of CdS/g-C3N4/TiO2/FTO shows diffraction peaks corresponding to the hexagonal wurtzite phase of CdS. Figure 3 shows the EDX mapping images of CdS/g-C3N4/TiO2 photoanode. The Sn comes from FTO substrate, and O is originated from FTO substrate and TiO2 nanorods. The same position of S and Cd indicates the CdS QD formation. The position distribution of C and N is similar, indicating the formation of g-C3N4 after spin coating. The EDX results are further confirmed by XPS.
The XPS survey in Fig. 4a exhibits that the existence of C, N, Cd, S, Ti, and O in the CdS/g-C3N4/TiO2 photoanode. The Ti 2p3/2 and 2p1/2 centered at 458.1 and 463.8 eV are in agreement with those of pure TiO2 (Fig. 4b) [22–25]. The C 1s shown in Fig. 3c has three peaks situated at 284.5, 288.4, and 285.6 eV, which corresponds to sp2 C–C bonds, sp2-bonded carbon in N–C=N, and sp3-bonded carbon species, respectively. For N, three peak signals of N1s located at 398.5, 400.1, and 401.1 eV are present and attributed to sp2 bond N in triazine rings, tertiary N in N-(C)3 units, respectively [26]. These results indicate the presence of graphite-like C3N4. Moreover, the Cd 3d-related peaks at 404.65 and 411.4 eV are observed and attributed to Cd 3d5/2 and Cd 3d3/2, respectively. The S2p XPS spectra can be separated to two peaks at 161.1 and 162.3 eV which are ascribed to S2− in CdS [27].
Figure 5 shows the comparison of the FTIR spectra of pure TiO2 nanorod and TiO2 nanorod/g-C3N4. The strong absorption between 500 to 800 cm−1 represents the bonds of Ti–O–Ti in both of the curves [28]. When g-C3N4 sheets are coated on TiO2 nanorods, several strong bands could be observed in the range of 1200–1700 cm−1 which are typical stretching modes of CN heterocycles [29]. Moreover, the peak at 813 cm−1 is due to variation of triazine units [30]. These absorption peaks once again confirm the existence of C3N4 on the as-prepared TiO2 nanorod photoanode.
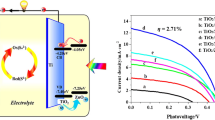
The cell performances are investigated as shown Fig. 6, and corresponding parameters are listed in Table 1. For both CdS/TiO2 and CdS/g-C3N4/TiO2 electrodes, the cell performances with different deposition cycles of CdS QDs are investigated. Both two kinds of electrodes exhibit the best performance with 10 cycles of CdS QD deposition, and the efficiency decreases with further increasing deposition cycles. This is probably due to the excessive deposition of QDs. If the deposition cycles of CdS QDs are more than 10, CdS QD with larger average size would be produced, and the aggregation and convergence among CdS QDs could happen at the surface of g-C3N4/TiO2. The larger CdS QDs would have poor ability to generate multiple excitons, originating from the disappearance of the quantum effect [31]. As shown in Table 1, the measurements of I-V characteristic indicate that the addition of g-C3N4 increases both the open circuit voltage and short circuit photocurrent density. As shown in Fig. 7, the photon-to-current conversion efficiency (IPCE) value is improved after coating g-C3N4 in the range of 300–600 nm. Compared with CdS/TiO2 electrode, it is worth noticing that the IPCE of CdS/g-C3N4/TiO2 electrodes is enhanced obviously between 400 and 500 nm. The maximum IPCE value occurs at ~470 nm which is very close to the bandgap of g-C3N4 used in this work. The improvement of IPCE could be due to the synergistic effect of g-C3N4 and CdS QD for sensitizing TiO2 nanorods.
The mechanism of the performance improvement of QDSSCs in this work is suggested as below. As illustrated in Fig. 8, a type II band alignment between TiO2 and g-C3N4 could be built due to suitable band structure of g-C3N4. Therefore, the immigration of photogenerated electrons from the conduction band (CB) of TiO2 and CdS QDs to g-C3N4 and electrolyte would be restrained. The g-C3N4 layer on TiO2 nanorods acted as both block layer and effective light absorption layer could effectively promote the electron transport by retarding the backward recombination of electrons from TiO2 and electrolyte and also contribute additional electrons to increase the electron concentration in the photoanodes, thus to enhance the performance of QDSSCs. Moreover, the synergistic effect of g-C3N4 and CdS QDs for sensitizing TiO2 nanorods would be the other reason. As shown in IPCE measurement, introducing g-C3N4 will further improve photoelectron injection to TiO2 particularly in the range of 400–500 nm, which suggests that the existence of g-C3N4 layer will supplement the adsorption of sunlight. The matching conduction bands and valence bands of g-C3N4 and TiO2 greatly enhanced the separation and transfer of the photogenerated electrons and holes in the composite; thus, the photoelectrochemical performance of the g-C3N4/TiO2 electrode is improved.
Conclusions
In summary, we introduce two-dimensional g-C3N4 layer in the single crystal TiO2 nanorod array photoanode. Compared with pure TiO2 nanorod array photoanodes, the g-C3N4 modified photoanodes showed an obvious improvement in cell performances, and a champion efficiency of 2.31 % was achieved, giving 23 % enhancement in cell efficiency. The improved performances were due to the matching conduction bands and valence bands of g-C3N4 and TiO2, which greatly enhanced the separation and transfer of the photogenerated electrons and holes and effectively suppressed interfacial recombination. Present work provides a new direction for improving the performance of QDSSCs.
Abbreviations
- CBD:
-
Chemical bath deposition
- DSSCs:
-
Dye-sensitized solar cells
- FESEM:
-
Field emission scanning electron microscopy
- FF:
-
Fill factor
- g-C3N4 :
-
Graphite carbon nitride
- QDs:
-
Quantum dots
- QDSSCs:
-
Quantum dot-sensitized solar cells
- SILAR:
-
Successive ionic layer adsorption and reaction
- TEM:
-
Transmission electron microscopy
- XPS:
-
X-ray photoelectron spectra
References
Chuang CM, Brown PR, Bulovic V, Bawendi MG (2014) Improved performance and stability in quantum dot solar cells through band alignment engineering. Nat Mater 13:796
Ren Z, Wang J, Pan Z, Zhao K, Zhang H, Li Y et al (2015) Amorphous TiO2 buffer layer boosts efficiency of quantum dot sensitized solar cells to over 9%. Chem Mater 27:8398
Weng ZY, Guo H, Liu XM, Wu SL, Yeung KW, Chu PK (2013) Nanostructured TiO2 for energy conversion and storage. RSC Adv 3:24758
Chen X, Jia B, Zhang Y, Gu M (2013) Exceeding the limit of plasmonic light trapping in textured screen-printed solar cells using Al nanoparticles and wrinkle-like graphene sheets. Light-Sci Appl 2:e92
Jiu JT, Isoda S, Wang FM, Adachi M (2006) Dye-sensitized solar cells based on a single-crystalline TiO2 nanorod film. J Phys Chem B 110:2087
Song MY, Ahn YR, Jo SM, Kim DY, Ahn J (2005) TiO2 single-crystalline nanorod electrode for quasi-solid-state dye-sensitized solar cells. Appl Phys Lett 87:113113
Zhang XY, Sun SH, Sun XJ, Zhao YR, Chen L, Yang Y (2016) Plasma-induced, nitrogen-doped graphene-based aerogels for high-performance supercapacitors. Light-Sci Appl 5:e16130
Wang H, Bai YS, Zhang H, Zhang ZH, Li JH, Guo L (2010) CdS quantum dots-sensitized TiO2 nanorod array on transparent conductive glass photoelectrodes. J Phys Chem C 114:16451
Bhande SS, Ambade RB, Shinde DV, Ambade SB, Patil SA, Naushad M et al (2015) Improved photoelectrochemical cell performance of tin oxide with functionalized-multiwalled carbon nanotubes-cadmium selenide sensitizer. ACS Appl Mater Interface 7:25094
Lee YL, Lo YS (2015) Highly efficient quantum-dot-sensitized solar cell based on co-sensitization of CdS/CdSe. Adv Funct Mater 19:604
Ayan-Varela M, Villar-Rodil S, Paredes JI, Munuera JM, Pagán A, Lozano-Pérez AA et al (2015) Investigating the dispersion behavior in solvents, biocompatibility, and use as support for highly efficient metal catalysts of exfoliated graphitic carbon nitride. ACS Appl Mater Interface 7:24032
Wang XC, Maeda K, Thomas A, Takanabe K, Xin G, Carlsson JM et al (2009) A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat Mater 8:76
Chen HM, Xie YH, Sun XQ, Lv ML, Wu FF, Zhang L et al (2015) Efficient charge separation based on type-II g-C3N4/TiO2-B nanowire/tubes heterostructure photocatalysts. Dalton Trans 44:13030
Low JX, Cao SW, Yu JF, Wageh S (2014) Two-dimensional layered composite photocatalysts. Chem Commun 50:10768
Wu DP, Cao K, Wang FJ, Wang HJ, Gao ZY, Xu F et al (2015) Two dimensional graphitic-phase C3N4 as multifunctional protecting layer for enhanced short-circuit photocurrent in ZnO based dye-sensitized solar cells. Chem Eng J 280:441
Xu J, Wang GX, Fan JJ, Liu BS, Cao SW, Yu JG (2015) gC3N4 modified TiO2 nanosheets with enhanced photoelectric conversion efficiency in dye-sensitized solar cells. J Power Sources 274:77
Kim H, Lee J, Yantara N, Boix PP, Kulkarni SA, Mhaisalkar S et al (2013) High efficiency solid-state sensitized solar cell-based on submicrometer rutile TiO2 nanorod and CH3NH3PbI3 perovskite sensitizer. Nano Lett 13:2412
LiaoYL ZSM, Ma J, Sun ZH, Yin C, Zhu CL et al (2014) Tailoring the morphology of g-C3N4 by self-assembly towards high photocatalytic performance. ChemCatChem 6:3419
Ge L, Han CC (2012) Synthesis of MWNTs/gC3N4 composite photocatalysts with efficient visible light photocatalytic hydrogen evolution activity. Appl Catal B 117:268
Dai K, Lu LH, Liang CH, Liu Q, Zhu GP (2014) Heterojunction of facet coupled gC3N4/surface-fluorinated TiO2 nanosheets for organic pollutants degradation under visible LED light irradiation. Appl Catal B 156:331
Baker DR, Kamat PV (2009) Photosensitization of TiO2 nanostructures with CdS quantum dots: particulate versus tubular support architectures. Adv Funct Mater 19:805
Chang F, Zhang J, Xie YC, Chen J, Li CL, Wang J et al (2014) Fabrication, characterization, and photocatalytic performance of exfoliated gC3N4−TiO2 hybrids. Appl Surf Sci 311:574
Wang JX, Huang J, Xie HL, Qu AL (2014) Synthesis of gC3N4/TiO2 with enhanced photocatalytic activity for H2 evolution by a simple method. Int J Hydrogen Energy 39:6354
Yan SC, Li ZS, Zou ZG (2009) Photodegradation performance of g-C3N4 fabricated by directly heating melamine. Langmuir 25:10397
Samanta S, Martha S, Parida K (2014) Facile synthesis of Au/g-C3N4 nanocomposites: an inorganic/organic hybrid plasmonic photocatalyst with enhanced hydrogen gas evolution under visible-light irradiation. ChemCatChem 6:1453
Li YG, Wei XL, Li HJ, Wang RR, Feng J, Yun H, Zhou AN (2015) Fabrication of inorganic–organic core-shell heterostructure: novel CdS@gC3N4 nanorod arrays for photoelectrochemical hydrogen evolution. RSC Adv 5:14074
Bai J, Li JH, Liu YB, Zhou BX, Cai WM (2010) A new glass substrate photoelectrocatalytic electrode for efficient visible-light hydrogen production: CdS sensitized TiO2 nanotube arrays. Appl Catal B 95:408
Mamakhel A, Tyrsted C, Bojesen ED, Hald P, Iversen BB (2013) Direct formation of crystalline phase pure rutile TiO2 nanostructures by a facile hydrothermal method. Cryst Growth Des 13:4730
Bojdys MJ, Müller JQ, Antonietti M, Thomas A (2008) Ionothermal synthesis of crystalline, condensed, graphitic carbon nitride. Chem Eur J 14:8177
Tan GQ, Li ZP, Yuan HY, Dan X (2014) Sorption of cadmium from aqueous solution with a highly effective sorbent-B-doped g-C3N4. Sep Sci Technol 49:1566
Su FL, Lu JW, Tian Y, Ma XB, Gong JL (2013) Branched TiO2 nanoarrays sensitized with CdS quantum dots for highly efficient photoelectrochemical water splitting. Phys Chem Chem Phys 15:12026
Acknowledgements
Financial support from the National Natural Science Foundation of China (authorization numbers: 61376020, 61574021) and the Science and Technology Department of Jilin Province (20130101009JC, 20140414024GH) is acknowledged.
Authors’ Contributions
WL and XL conceived the idea. QG carried out the experiments. SS, XZ, and LD took part in the experiments and the discussion of the results. WL, QG, and XL drafted the manuscript. All authors read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Gao, Q., Sun, S., Li, X. et al. Enhancing Performance of CdS Quantum Dot-Sensitized Solar Cells by Two-Dimensional g-C3N4 Modified TiO2 Nanorods. Nanoscale Res Lett 11, 463 (2016). https://doi.org/10.1186/s11671-016-1677-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11671-016-1677-1