Abstract
The rational design and preparation of hierarchical nanoarchitectures are critical for enhanced photocatalytic hydrogen evolution reaction (HER). Herein, well-integrated hollow ZnO@TiO2 heterojunctions were obtained by a simple hydrothermal method. This unique hierarchical heterostructure not only caused multiple reflections which enhances the light absorption but also improved the lifetime and transfer of photogenerated charge carriers due to the potential difference generated on the ZnO–TiO2 interface. As a result, compared to bare ZnO and TiO2, the ZnO@TiO2 composite photocatalyst exhibited higher hydrogen production rated up to 0.152 mmol h−1 g−1 under simulated solar light. In addition, highly repeated photostability was also observed on the ZnO@TiO2 composite photocatalyst even after a continuous test for 30 h. It is expected that this low-cost, nontoxic, and readily available ZnO@TiO2 catalyst could exhibit promising potential in photocatalytic H2 to meet the future fuel needs.
Similar content being viewed by others
Background
Hydrogen (H2), one of the most important clean and sustainable energy, has been regarded as a promising alternative energy for meeting future fuel needs [1,2,3,4,5]. Since the discovery of photoelectrochemical (PEC) water-splitting system by Fujishima and Honda in the 1970s [6], the production of H2 based on TiO2 semiconductor photocatalysts using sunlight has attracted increasing attention. However, the practical application of single bare TiO2 in the industry is still a challenge due to the high-rate recombination of photogenerated electrons and holes at the surface of TiO2 results in a low quantum efficiency. To date, many efforts have been made to design TiO2-based composite photocatalysts to solve the above issues, such as coupling with another semiconductor, doping transition metal ions or nonmetal atoms, and so on [7,8,9]. In particular, the formation of semiconductor–semiconductor heterojunctions with matching band potentials is an effective way to prevent the charge recombination and increase the lifetime of the charge carriers [10,11,12].
Among the various semiconductors, ZnO is also extensively studied because of its identical properties of TiO2 with non-toxicity, cheapness, high efficiency, and chemical stability [13, 14]. Since the conduction band (CB) and valence band (VB) of ZnO lie above those of TiO2, the photogenerated electrons in ZnO will be transferred to TiO2 once a heterojunction was formed between TiO2 and ZnO. This kind of ZnO@TiO2 composite heterojunction will benefit for the separation of photogenerated electron–hole pairs, thus leading more electrons accumulated on the TiO2 which will react with H2O to generate H2 [15,16,17].
In addition to the above we have discussed, geometric shapes and morphologies of the photocatalysts also heavily influence the hydrogen evolution reaction (HER) performance [18,19,20]. It is has been reported that the diffractions on the hollow spheres and the multiple reflections due to the shell structure would enhance the effectiveness of light utilization [21]. For example, Li’s group prepared hydrogenated cage-like titania hollow spheres exhibited much higher HER activities than solid structure [22]. Beyond that, the spherical hollow structures have the advantages of large specific surface area, reduced transport lengths for charge carriers, and good chemical and thermal stability, which all contribute to the excellent photocatalytic ability [23]. However, most of the research has focused on the preparation of composite hollow spheres by doping transition element, such as Ce–ZnO [24], Ni–ZnO [25], Ag–TiO2 [26], Au–TiO2 [27], and so on. To the best of our knowledge, few studies reported on the synthesis of closed, complete, and intact hollow spheres composed of mixed metal oxides porous particles. Even so, most of these composites are applied in photocatalytic degradation of organic pollutants but not in the photocatalytic hydrogen production.
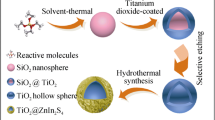
In this paper, we reported a facile method to synthesize hierarchically porous ZnO@TiO2 composite hollow microspheres and applied them in the photocatalytic H2. The hollow spheres enhanced the light absorption by multiple reflections, at the same time, the lifetime and transfer rate of photogenerated charge carriers were also improved due to the potential difference generated on the ZnO–TiO2 interface. The result showed that the ZnO@TiO2 composite photocatalyst exhibited enhanced H2 evolution rate, compared to the bare ZnO and TiO2. In addition, the mechanism of the photocatalytic H2 on the ZnO@TiO2 composite hollow spheres was discussed in detail.
Methods
Synthesis of the Hierarchical ZnO@TiO2 Hollow Spheres
The preparation of ZnO@TiO2 composites was based on a very facile one-step template-free hydrothermal method at ambient conditions. In a typical procedure, 0.015 mol of Ti(SO4)2, 0.015 mol of Zn(NO3)2·6H2O, 0.015 mol of NH4F, and 0.06 mol of CO(NH2)2 were added to a beaker with 50 mL deionized water. After stirring for 60 min, the mixture solution was transferred into a Teflon-lined stainless steel autoclave and heated in an electric oven at 180 °C for 12 h. After that, the white precipitate was thoroughly washed with ethanol four times and then dried at 60 °C for 12 h to obtain ZnO@TiO2 heterostructures. For comparison, bare TiO2 and ZnO were prepared under the same conditions.
Synthesis of Pt–ZnO@TiO2 Samples
In a typical synthesis process of Pt–ZnO@TiO2 samples, the ZnO@TiO2 hollow spheres were put into a container containing 10 vol% triethanolamine and H2PtCl6 solution. Then, the system was bubbled with nitrogen for 30 min to remove the air. Finally, the Pt was in situ photodeposited on the ZnO@TiO2 hollow spheres under a full arc light irradiation (λ > 300 nm) for 2 h. The Pt content can be tuned by the concentration of H2PtCl6 and the reaction time, which was determined by inductively coupled plasma (ICP, PE5300DV).
Characterization
The morphology of ZnO@TiO2 heterostructures was characterized via field emission scanning electron microscope (FESEM, Hitachi, Japan), transmission electron microscopy (TEM, Tecnai F20), high-angle annular dark field scanning TEM (STEM, Tecnai F20), and high-resolution TEM (HRTEM, Tecnai F20). The energy-dispersive X-ray spectroscopy (EDS) mapping images were captured on a Tecnai G2 F20 S-TWIN atomic resolution analytic microscope. The crystal phase properties of the samples were characterized using an X-ray diffractometer with Cu–K radiation (XRD, M21X, MAC Science Ltd., Japan). The BET specific surface areas were measured on Belsorp-mini II analyzer (Japan).
Photoelectrochemical Measurements
Photocurrent studies were performed on a CHI 660D electrochemical workstation, using a three-electrode configuration where fluorine-doped tin oxide (FTO) electrodes deposited with the samples as working electrode, Pt as counter electrode, and a saturated calomel electrode (SCE) as reference. The electrolyte was 0.35 M/0.25 M Na2S–Na2SO3 aqueous solution. For the fabrication of the working electrode, 0.25 g of the sample was grinded with 0.06 g polyethylene glycol (PEG, molecular weight 20,000) and 0.5 mL ethanol to make a slurry. Then, the slurry was spread onto a 1 × 4 cm FTO glass by the doctor blade technique and then allowed to dry in air. A 300 W xenon arc lamp served as a simulated solar light irradiation source (Perfectlight, PLS-SXE 300C, Beijing, China). The incident light intensity was tuned to be 100 mW/cm2 measured by NOVA Oriel 70260 with a thermodetector.
Photocatalytic Hydrogen Production Tests
Photocatalytic hydrogen production experiments were performed in a sealed quartz flask at ambient temperature and under atmospheric pressure. A 300 W xenon arc lamp (Perfect light, PLS-SXE 300C, Beijing, China) was used as the light source to trigger the photocatalytic reaction. The evolved H2 were collected and online-analyzed by a H2-solar system (Beijing Trusttech Technology Co., Ltd.) with a gas chromatogram equipped with a thermal conductivity detector (TCD), 5A molecular sieve column, and nitrogen as the carrier gas. All photocatalytic experiments over 100 mg photocatalyst were performed in an aqueous solution containing H2O (80 mL) and alcohol (20 mL). Prior to irradiation, the system was deaerated by bubbling nitrogen for 15 min. During the photocatalytic reaction, the reactor was tightly sealed to avoid gas exchange.
Results and Discussion
The size and morphology of the as-prepared ZnO@TiO2 hollow spheres were displayed in Fig. 1. Figure 1a shows the sample has a uniform spherical morphology with a mean diameter about 1.45 μm according to the nanoparticle size distribution (inset of Fig. 1a). Figure 1b reveals a single broken sphere, indicating that the prepared sample is a hollow structure composed of small particles. TEM image was further used to confirm the structure of the ZnO@TiO2 hollow spheres. The color change of the ZnO@TiO2 spheres at the center and the outside realm was dark and bright, respectively, confirming the ZnO@TiO2 spheres were hollow structure (Fig. 2a). A high-magnified view in Fig. 2b also depicts the surface of the hollow spheres was rough which were constructed by nanoparticles subunits, as a result in the formation of the hierarchical heterostructure of ZnO@TiO2 hollow spheres. The elemental maps in Fig. 2(d–f) were used to confirm the elemental distribution in the ZnO@TiO2 hollow spheres. It can be seen that the Zn, Ti, and O were uniformly distributed in ZnO@TiO2 hollow spheres.
HRTEM images in Fig. 3 verified the heterojunction structure of ZnO@TiO2 hollow spheres. The selected areas in Fig. 3a marked by white square were magnified in Fig. 3b–d, corresponding to ZnO, TiO2, and ZnO@TiO2 heterojunction. The lattice spacing distances of 0.28 and 0.35 nm were corresponding to the (100) planes of wurtzite ZnO and (101) planes of the anatase TiO2, respectively, as shown in Fig. 3b, c. Figure 3d shows a clear transition from wurtzite ZnO phase to anatase TiO2 phase, which confirmed the heterojunction was formed at the interface between ZnO and TiO2. Such heterojunction structure can greatly promote the photoexcited electron transfer for enhanced photocatalytic activity.
The pore structure properties of ZnO, TiO2, and ZnO@TiO2 samples were further determined by the N2 adsorption–desorption isotherms and corresponding Barrett–Joyner–Halenda (BJH) pore size distribution plots (Fig. 4). All the samples showed a type IV isotherm with a hysteresis loop at a high relative pressure (P/P 0 > 0.7), demonstrating the existence of mesoporous structures according to International Union of Pure and Applied Chemistry (IUPAC) classification. The inset of Fig. 4 is BJH pore size distribution plots, which further indicated that all the samples have the mesoporous structures. Meanwhile, the calculated BET surface areas of the ZnO@TiO2 microsphere was about 102 m2 g−1, which was much larger than that of ZnO (23 m2 g−1) and TiO2 (35 m2 g−1). It can be concluded the introduction of ZnO into TiO2 to form the ZnO@TiO2 hollow spheres could increase the surface areas greatly, although all the samples have the mesoporous structures. The higher surface areas of ZnO@TiO2 hollow spheres would provide more sites for enhanced catalytic H2 performance.
The photocatalytic ability of the as-prepared samples was evaluated by photocurrent and photocatalytic H2 tests. As shown in Fig. 5a, the ZnO@TiO2 hollow spheres yielded the highest photocurrent density of 3.38 mA/cm2, which was more than 2.61, 2.17 times higher than that of ZnO and TiO2, respectively. These results mean the stronger ability of producing charge carriers and improved separation efficiency of ZnO@TiO2 hollow spheres. As excepted, the hydrogen production rate of ZnO@TiO2 hollow spheres reached to 0.152 mmol h−1 g−1, higher than the 0.039 mmol h−1 g−1 of ZnO and 0.085 mmol h−1 g−1 of TiO2 (Fig. 5b). Pt, as a very high-efficiency noble metal cocatalyst, has been widely used for H2 evolution reaction in the reported literature [8, 11]. A series of Pt–ZnO@TiO2 with different Pt contents were prepared and compared in Fig. 5c. It was shown that the loading of Pt onto ZnO@TiO2 hollow spheres could significantly enhance the H2 evolution activity and the sample with 1.5 at % Pt exhibiting the highest H2 evolution rate. Figure 5d shows that the ZnO@TiO2 hollow spheres still retained its original photocatalytic activity without noticeable degradation in the five reaction cycles for 30 h, which demonstrates the exceptional photocatalytic stability.
a Photocurrent responses and b photocatalytic H2 evolution of bare ZnO, bare TiO2, and ZnO@TiO2 heterojunctions. c Photocatalytic H2 evolution over Pt–ZnO@TiO2 heterojunctions composites with different weight ratios of Pt. d Photocatalytic stability of ZnO@TiO2 hollow spheres. All the measurement was carried out under a simulated solar light irradiation source with intensity of 100 mW/cm2
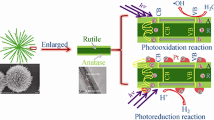
A photocatalytic mechanism was proposed for the improved HER activity of the ZnO@TiO2 hollow spheres, as shown in Fig. 6. Under simulated solar light irradiation, the electrons of both ZnO and TiO2 were excited from their valence bands (VB) to their conduction bands (CB). Since the conduction band (CB) and valence band (VB) of ZnO were more positive than those of TiO2, the photogenerated electrons transferred from ZnO to TiO2 through the intimate interfacial contacts [16]. Then, the more accumulated electrons on the TiO2 reacted with H2O for generating H2 for the higher photocatalytic H2 rate (as shown on the right of Fig. 6). At the same time, the photogenerated holes in the VB of TiO2 migrated to ZnO, which were trapped by the sacrificial agent to keep the thermodynamical balance. Additionally, the hierarchical hollow spheres benefit for light scatter and multiple reflections among ZnO@TiO2 composite photocatalyst, which would enhance the effectiveness of light utilization [10, 21, 22]. Thus, more free electrons and holes would be generated due to the increased effective photon path length [21, 22], leading to a higher HER efficiency (as shown in the left of Fig. 6).
Conclusions
In summary, the hierarchical heterostructure of ZnO@TiO2 hollow spheres has been successfully prepared via a simple hydrothermal method. Compared to bare ZnO and TiO2, the ZnO@TiO2 composite photocatalyst exhibited high hydrogen production rated up to 0.152 mmol h−1 g−1 under simulated solar light. It is believed that hierarchical heterostructure increased the surface area which proving more active sites for effective HER and simultaneously improved the lifetime and transfer of photogenerated charge carriers due to the potential difference generated on the ZnO–TiO2 interface. Moreover, the ZnO@TiO2 composite photocatalyst exhibited good durability even after being reused five times. This work demonstrated a good prospect for photocatalytic H2 evolution from water based on the rational use and preparation of high activity, inexpensive, and chemical stability of ZnO and TiO2.
References
Zhou W, Li W, Wang JQ et al (2014) Ordered mesoporous black TiO2 as highly efficient hydrogen evolution photocatalyst. J Am Chem Soc 136:9280–9283
Li Y, Wang LL, Zhang SQ et al (2017) Cracked monolayer 1T MoS2 with abundant active sites for enhanced electrocatalytic hydrogen evolution. Catal Sci Technol 7:718–724
Zhang Y, Fu LJ, Shu Z et al (2014) Substitutional doping for aluminosilicate mineral and superior water splitting performance. Nanoscale Res Lett 12:456
Zhao L, Jia J, Yang Z et al (2017) One-step synthesis of CdS nanoparticles/MoS2 nanosheets heterostructure on porous molybdenum sheet for enhanced photocatalytic H2 evolution. Appl Catal B Environ 210:290–296
Li Y, Wang LL, Cai T et al (2017) Glucose-assisted synthesize 1D/2D nearly vertical CdS/MoS2 heterostructures for efficient photocatalytic hydrogen evolution. Chem Eng J 321:366–374
Fujishima A, Honda K (1972) Electrochemical photolysis of water at a semiconductor electrode. Nature 238:37–38
Park JH, Kim S, Bard AJ (2006) Novel carbon-doped TiO2 nanotube arrays with high aspect ratios for efficient solar water splitting. Nano Lett 6:24–28
Loget G, Schmuki P (2014) H2 mapping on Pt-loaded TiO2 nanotube gradient arrays. Langmuir 30:15356–15363
Zhou W, Yin Z, Du Y et al (2013) Synthesis of few-layer MoS2 nanosheet-coated TiO2 nanobelt heterostructures for enhanced photocatalytic activities. Small 9:140–147
Wang LL, Duan X, Wang G et al (2016) Omnidirectional enhancement of photocatalytic hydrogen evolution over hierarchical “cauline leaf” nanoarchitectures. Appl Catal B Environ 186:88–96
Park H, Kim YK, Choi W (2011) Reversing CdS preparation order and its effects on photocatalytic hydrogen production of CdS/Pt-TiO2 hybrids under visible light. J Phys Chem C 115:6141–6148
Liu CB, Wang LL, Tang YH et al (2015) Vertical single or few-layer MoS2 nanosheets rooting into TiO2 nanofibers for highly efficient photocatalytic hydrogen evolution. Appl Catal B Environ 164:1–9
Zhang B, Wang F, Zhu C et al (2015) A facile self-assembly synthesis of hexagonal ZnO nanosheet films and their photoelectrochemical properties. Nano-Micro Lett 8:137–142
Kayaci F, Vempati S, Ozgit-Akgun C et al (2015) Transformation of polymer-ZnO core–shell nanofibers into ZnO hollow nanofibers: intrinsic defect reorganization in ZnO and its influence on the photocatalysis. Appl Catal B Environ 176:646–653
Li X, Lv K, Deng K et al (2009) Synthesis and characterization of ZnO and TiO2 hollow spheres with enhanced photoreactivity. Mater Sci Eng B 158:40–47
Agrawal M, Gupta S, Pich A et al (2009) A facile approach to fabrication of ZnO–TiO2 hollow spheres. Chem Mater 21:5343–5348
Wang Y, Zhu S, Chen XY et al (2014) One-step template-free fabrication of mesoporous ZnO/TiO2 hollow microspheres with enhanced photocatalytic activity. Appl Surf Sci 307:263–271
He H, Lin J, Fu W et al (2016) MoS2/TiO2 edge-on heterostructure for efficient photocatalytic hydrogen evolution. Adv Energy Mater 6:1600464
Wang L, Li Y, Liu Y (2017) Reduced graphene oxide@TiO2 nanorod@reduced graphene oxide hybrid nanostructures for photoelectrochemical hydrogen production. Micro Nano Lett 7:494–496
Wang L, Liu X, Luo J et al (2017) Self-optimization of the active site of molybdenum disulfide by an irreversible phase transition during photocatalytic hydrogen evolution. Angew Chem Int Ed 129:7718–7722
Kondo Y, Yoshikawa H, Awaga K et al (2008) Preparation, photocatalytic activities, and dye-sensitized solar-cell performance of submicron-scale TiO2 hollow spheres. Langmuir 24:547–500
Wang Y, Cai J, Wu M et al (2016) Hydrogenated cagelike titania hollow spherical photocatalysts for hydrogen evolution under simulated solar light irradiation. ACS Appl Mater Interfaces 8:23006–23014
Zhang C, Zhou Y, Zhang Y et al (2017) Double-shelled TiO2 hollow spheres assembled with TiO2 nanosheets. Chem Eur J 23:4336–4343
Jiang J, Zhang K, Chen X et al (2017) Porous Ce-doped ZnO hollow sphere with enhanced photodegradation activity for artificial waste water. J Alloy Compd 699:907–913
Wang Y, Liu T, Huang Q et al (2016) Synthesis and their photocatalytic properties of Ni-doped ZnO hollow microspheres. J Mater Res 31:2317–2328
Xiang Q, Yu J, Cheng B et al (2010) Microwave-hydrothermal preparation and visible-light photoactivity of plasmonic photocatalyst Ag-TiO2 nanocomposite hollow spheres. Chem-Asian J 5:1466–1474
Dinh CT, Yen H, Kleitz F (2014) Three-dimensional ordered assembly of thin-shell Au/TiO2 hollow nanospheres for enhanced visible-light-driven photocatalysis. Angew Chem Int Ed 53:6618–6623
Funding
This research was supported by the National Natural Science Foundation of China (No. 51608175), the Key Project of Science and Technology of the Education Department of Henan Province (No. 17B610004), and the Doctor Foundation of Henan Institute of Engineering (Nos. D2015020, D2014021).
Author information
Authors and Affiliations
Contributions
The concept was developed by WLL and LY. This manuscript was written by LY and WLL. The experiment and the data analysis were carried out by GFX, WLL, and LY. The preparation of samples is performed by LJ, DP, and YT. The characterization of samples are made by YK and GFX. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Li, Y., Wang, L., Liang, J. et al. Hierarchical Heterostructure of ZnO@TiO2 Hollow Spheres for Highly Efficient Photocatalytic Hydrogen Evolution. Nanoscale Res Lett 12, 531 (2017). https://doi.org/10.1186/s11671-017-2304-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11671-017-2304-5