Abstract
Conductive gels are a special class of soft materials. They harness the 3D micro/nanostructures of gels with the electrical and optical properties of semiconductors, producing excellent novel attributes, like the formation of an intricate network of conducting micro/nanostructures that facilitates the easy movement of charge carriers. Conductive gels encompass interesting properties, like adhesion, porosity, swelling, and good mechanical properties compared to those of bulk conducting polymers. The porous structure of the gels allows the easy diffusion of ions and molecules and the swelling nature provides an effective interface between molecular chains and solution phases, whereas good mechanical properties enable their practical applications. Due to these excellent assets, conductive gels are promising candidates for applications like energy conversion and storage, sensors, medical and biodevices, actuators, superhydrophobic coatings, etc. Conductive gels offer promising applications, e.g., as soft sensors, energy storage, and wearable electronics. Hydrogels with ionic species have some potential in this area. However, they suffer from dehydration due to evaporation when exposed to the air which limits their applications and lifespan. In addition to conductive polymers and organic charge transfer complexes, there is another class of organic matter called “conductive gels” that are used in the organic nanoelectronics industry. The main features of this family of organic materials include controllable photoluminescence, use in photon upconversion technology, and storage of optical energy and its conversion into electricity. Various parameters change the electronic and optical behaviors of these materials, which can be changed by controlling some of the structural and chemical parameters of conductive gels, their electronic and optical behaviors depending on the applications. If the conjugated molecules with π bonds come together spontaneously, in a relative order, to form non-covalent bonds, they form a gel-like structure that has photoluminescence properties. The reason for this is the possibility of excitation of highest occupied molecular orbital level electrons of these molecules due to the collision of landing photons and their transfer to the lowest unoccupied molecular orbital level. This property can be used in various nanoelectronic applications such as field-effect organic transistors, organic solar cells, and sensors to detect explosives. In this paper, the general introduction of conductive or conjugated gels with π bonds is discussed and some of the physical issues surrounding electron excitation due to incident radiation and the mobility of charge carriers, the position, and role of conductive gels in each of these applications are discussed.
Similar content being viewed by others
Introduction
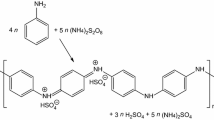
The organs of some marine animals have structures rich in water and organic matter. For example, mermaid tissues are composed of 1% organic matter in 96% water and are a good example of functional hydrogels. Chemists and materials engineers have long sought to mimic nature and make materials with the function of natural hydrogels. “Gels” are examples of these materials that can trap very large volumes of solutions in their three-dimensional lattice-like structure. These semisolid viscoelastic materials are used today in various parts of human life, including food and cosmetics. The process of gelation or synthesis of hydrogels is accelerated by the formation of non-covalent bonds (i.e., hydrogen bonds, ionic bonds, π bonds, and van der Waals bonds in low molecular weight organic molecules) [1,2,3]. “Conjugated polymers with π-bonds” are another class of materials that are widely used in optoelectronic applications. The physical foundations, electronic properties, and nanoelectronic applications of this family of materials have been studied in detail in other articles on the Nano Education site. As mentioned, conductive polymers are often used as main components in organic electronics such as light-emitting diodes (LEDs), organic field-effect transistors (OFETs), and organic solar cells. Organic solar cells (OSCs) are used. The performance and efficiency of these devices are controlled by the degree of crystallinity of these materials in the process of making thin layers. Controlling the morphology and crystallinity of conductive polymers along with limited access to high purity in the synthesis process and low solubility of conjugated polymers in aqueous solutions is one of the problems and challenges facing the organic electronics industry. These challenges often lead to the formation of undesirable arrays of organic and non-uniform molecules of chemical composition and crystal structure. To solve these problems, “supramolecular polymers made of conjugated molecules with π bonds” have been developed [4,5,6]. The formation of these large molecule polymers under certain conditions leads to the formation of conductive or conjugated gels with π bonds (π gels). In fact, the molecular structure of conductive gels consists of regular arrays of conjugated molecules of various shapes and dimensions [7,8,9,10,11]. Conductive gels, due to the dynamic nature of molecular arrays, have the ability to regulate some electronic properties such as photoluminescence, mobility of charge carriers, and electrical conductivity. In this paper, recent developments in the field of conductive gels and their applications in the field of nanoelectronics will be reviewed [12,13,14,15,16,17]. Many conjugated organic materials with π bonds have a wide barrier band and exhibit semiconductor behaviors when doped with appropriate materials [18,19,20,21,22]. Tetrathiafulvalene (TTF) and its derivatives are an exception to this rule and, if properly doped, show very high electrical conductivity [23,24,25,26]. In recent years, some researchers have sought to make conductive gels based on TTF in order to obtain a very high electrical conductivity [27,28,29,30]. The chemical structure of this substance is shown in Fig. 1 (Structure 1) [31]. The current curve according to the voltage of this material, if not doped with a suitable additive, is in the form of a yellow horizontal line, which is shown in Fig. 1b. This curve shows that with any voltage difference, the current flowing through the material is very small. On the other hand, by doping compound I2 at room temperature, the electrical conductivity of these materials is improved. A new solution to increase the conductivity of these materials is to bind gold nanoparticles to the ligands of TTF molecules. This is shown in Fig. 1 as structures 16 and 17. As can be seen, the addition of gold nanoparticles leads to metallic behaviors in TTF-based conductive gels. In general, in order to be able to use conductive gels in the manufacture of nanoelectronic devices, the method of doping or adding conductive nanoparticles is very helpful [32,33,34,35,36]. As another practical example, large molecules of conductive gels can be produced as nanofibers after doping and their conductivity behaviors in different molecular arrangements can be investigated [37,38,39,40,41,42]. For example, Fig. 2 shows two different molecular structures in oligothiophene-based conductive gels that are produced as continuous, cohesive fibers. The current–voltage diagram of these nanofibers shows that the molecular structure and chemical composition of large molecules and their self-arrangement strongly affect the electrical resistance of these materials. Therefore, to achieve the highest electrical conductivity in conductive gels, control of the spatial arrangement of molecules is very important [43,44,45]. Table 1 shows the summary of properties and nanoelectronic applications of conductive gels.
a Molecular structure of tetrathiafulvalene (TTF)-based conductive gels; b current–voltage diagrams obtained by atomic force microscopy (AFM) for conductive gels in the presence and absence of structures 2 and 3 and doping conditions with I2 [37]
a Two types of molecular structures of oligothiophene-based conductive gels; b synthesis of aligned nanofibers from conductive gels; c current–voltage diagrams of structures 19a and 19b in both self-arranged and non-self-arranged states [56]
Conductive polymers have been researched over the past few decades owing to their unique ability to provide tunable electrical conductivity and flexibility during processing [46,47,48]. The conductivity of conductive polymers depends on the molecular structures of the constituent materials, the level of doping, and the ordering of the molecular packing. With the rapid emergence of nanoscience and nanotechnology, it is anticipated that conductive polymers with well-defined nanostructures can translate the properties of their bulk forms and exhibit unusual chemical/physical properties because of the confined dimensions of the nanomaterials [49,50,51,52,53,54].
Conductive polymers with various nanostructures including 0D nanoparticles, [55] 1D nanofibers [56, 57], and 2D nanosheets [58] have been developed and applied in a range of technological areas, such as sensors, electronics, and energy storage and conversion devices. However, the electrical properties of these nanostructured conductive polymers could be weakened by structural defects induced by inhomogeneous aggregation, severe restacking, and poor contacts during processing and assembly [59]. The development of nanostructured conductive polymers with tunable microstructures and controllable chemical/physical properties still remains a challenge [60,61,62,63].
Inspired by the chemical/structural features and synthetic approaches of natural gels [64], conductive polymer gels (CPGs) with 3D networked structures were recently developed by cross-linking the conjugated polymer chains using molecules with multiple functional groups [65]. CPGs show the unique features of gel materials: They are dilute cross-linked systems and exhibit no flow when in a steady state. This monolithic structure inherits the conductive properties of the conjugated polymeric chains and generates highly tunable chemical/physical properties derived from its cross-linked network [2, 48, 50,51,52,53,54, 60,61,62,63, 66,67,68,69,70], including flexibility, stretchability, ionic conductivity, electrochemical activity, and so forth. Meanwhile, CPGs have emerged as a unique material platform to develop functional materials by building interpenetrating structures with a second polymeric network, loading specific nanoparticles, or serving as a precursor for graphitic carbon frameworks [71].
Conductive hydrogels have drawn significant attention in the field of stretchable/wearable sensors due to their intrinsic stretchability, tunable conductivity, biocompatibility, multi-stimuli sensitivity, and self-healing ability. Recent advancements in hydrogel- and organohydrogel-based sensors, including a novel sensing mechanism, outstanding performance, and broad application scenarios, suggest the great potential of hydrogels for stretchable electronics. However, a systematic summary of hydrogel- and organohydrogel-based sensors in terms of their working principles, unique properties, and promising applications is still lacking. In this spotlight, we present recent advances in hydrogel- and organohydrogel-based stretchable sensors with four main sections: improved stability of hydrogels, fabrication and characterization of organohydrogel, working principles, and performance of different types of sensors. We particularly highlight our recent work on ultrastretchable and high-performance strain, temperature, humidity, and gas sensors based on polyacrylamide/carrageenan double-network hydrogel and ethylene glycol/glycerol modified organohydrogels obtained via a facile solvent displacement strategy. The organohydrogels display higher stability (drying and freezing tolerances) and sensing performances than corresponding hydrogels. The sensing mechanisms, key factors influencing the performance, and application prospects of these sensors are revealed. Particularly, we find that the hindering effect of polymer networks on the ionic transport is one of the key mechanisms applicable for all four of these kinds of sensors [12].
Electronic Properties of Conductive Gels
Chromophore is the part of a molecule that causes color in it. Chromaticity occurs when matter can absorb a certain wavelength of visible light and pass or reflect the rest. A chromophore is a region of a molecule where the energy balance difference between two molecular orbitals is within the energy range of part of the incident beam spectrum [56]. A certain wavelength of light due to a collision with a molecule can be absorbed by its electrons and excite them from the ground state to the excited state. What is very important in conductive gels and affects the efficiency of absorption of light energy by conductive gels is the arrangement and spatial arrangement of chromophores giving or receiving electron with optimal distance and orientation. To date, a wide range of conductive gels have been developed as high-performance scaffolds for energy transfer or conversion. In each case, they have seized it, despite obstacles we can scarcely imagine. In other words, it is generally preferred that the chromophores giving and receiving conductive gels can be arranged on their own and that the electron excitation properties of these materials due to light beam can be optimized by controlling the molecular self-arranging property and increasing the energy conversion efficiency [57]. In other words, by controlling the amount of energy transfer between the donor and electron acceptor molecules in the three-dimensional structure and network like conductive gels, the optical energy absorption or optical behavior of the material can be adjusted and used to make optical emitting devices (LEDs). In order to be able to use conductive gels in the nanoelectronics and organic electronics industries, it is necessary for the three-dimensional structure of these materials to be able to transfer excited electrons at a macroscopic distance. The emergence of this feature requires regular arrangement of electron donor and receiver centers in different directions. In practice, the self-arrangement of donor and receptor molecules does this. Figure 3 shows a diagram of the molecular structure of multi-chromophore conductive gels arranged in self-assembled nanofibers. The core of each nanofiber is composed of an elongated chain of conjugated molecules with π bonds that, with their regular arrangement, lead to the formation of a continuous structure. The gel changes color when exposed to ultraviolet light and its macroscopic appearance changes from dark orange to yellow. The reason for this discoloration is the excitation of electron donor and electron acceptor chromophores by the collision of ultraviolet light and their movement in the three-dimensional structure of the gel. Today, photon upconversion is one of the most important applications of electron excitation in conductive gels. In this phenomenon, several weak and low-energy photons are absorbed by the material due to electron excitation, and in return, a higher-energy photon is emitted by the material. It has been observed that in conductive gels, the electron donor centers can be combined in a polymer field containing electron acceptor centers; with a special arrangement, the photon upconversion property in the gel can be achieved. Take Fig. 4, for example. In this form, the conducting gel of 9,10-diphenylanthracene as the electron receptor is doped by Pt (II) octaethylporphyrin (PtOEP) as the electron donor. As can be seen, the collision of the incident beam leads to the excitation of electrons in the donor centers and the transfer of charge carriers to the recipient centers. Finally, matter emits a higher energy beam for several electrons excited at a lower energy [58]. The photoluminescence spectrum diagram of this conductive gel shows that the output beam has a relatively wide range of wavelengths in the range of 400 to 475 nm.
a Molecular structure of a conductive gel with electron donor and electron acceptor chromophores; b a nanofiber whose core consists of a regular array of electron donor and electron acceptor chromophores. These molecules are sensitive to ultraviolet light, and when the beam hits the gel, electron excitation occurs and part of the input spectrum is absorbed by the material. Absorption of this spectrum of light causes the color of this material to change; c the macroscopic image of the gel which has changed color due to contact with ultraviolet light [1, 2]
Molecular structures: a conductive gels and electron donor centers; b a schematic of the specific spatial arrangement of the two-phase structure of the conductive gel–electron donor centers after doping; the photon upconversion phenomenon is shown schematically; c the photoluminescence spectrum of a conductive gel doped with electron donor centers; and the emission of light output is done in the wavelength range of 400 to 475 nm. In this study, 9,10-diphenylanthracene acted as conductive gels (electron receptor centers) and PtOEP acted as electron donor centers [3]
Electron Transfer Due to Optical Excitation
In addition to controllable photoluminescence behavior, one of the main approaches to the development of conjugated gels with π bonds is the conversion of optical energy into electrical energy by electron excitation. In other words, scientists are looking for conductive gels to emit their excited electrons through an electronic circuit, instead of emitting light or exhibiting photon upconversion due to electron stimulation after exposure to the incoming beam. Collected and stored. Observations show that conductive gels are a good option for converting solar energy into electric current, because the electron donor and receiver centers in the three-dimensional structure of these materials are arranged independently and in a controllable arrangement, and the possibility of electron transfer at macroscopic distances [59, 72]. There is: One method of producing conductive gel-based systems to convert solar energy into electricity is to functionalize conductive gels with electron donor–receiver units. What is very important in these gel structures is to prevent the recombination of electrons, which is also done by choosing the right chromophores for giving and receiving electrically charged carriers. Recently, efforts have been made to design and synthesize gels with optimal conversion and storage properties of electric current through visible light absorption. One of these systems is a gel-like structure, which is schematically shown in Fig. 5. In this gel, naphthalimide as electron donor and perylene monoimide as electron receptor are dissolved in hexane/dichloromethane solution and then functionalized with α,β-dihydroxypropyl chains. The functionalization of these compounds leads to the self-assembly of these molecules and the formation of regular molecular structures within the solution at low concentrations (Fig. 5a). As the concentration of the solution increases, non-covalent bonds predominate and the cell turns into a gel (Fig. 5b). It has been observed that at concentrations above a critical limit, a nanostructured gel is formed in which the charge carriers generated by the excitation of visible light are transferred from the naphthalimide unit to the perylene monoimide unit, and thus, the electric current in it flows inside the network structure like a conductive gel. Also, if indole is used as the electron donor, the electron transfer process will be accelerated and the electron path will be from naphthalimide to indole and finally to perylene monoimide, respectively. It should be noted that in biphasic conducting gels, the rate of electron–hole recombination is much lower than the rate of electron production due to light excitation, and the reason for this is the self-assembly arrangement of the large molecules that make up the gel. Such a stable transfer from charge carriers can be used in water splitting and artificial photosynthesis.
a, b. The molecular structure of a conductive gel synthesized from naphthalimide as the electron donor and perylene monoimide as the electron acceptor in a hexane/dichloromethane solution medium to enhance the electron, transfer kinetics in the three-dimensional structure of the gel, Indole is also used as an intermediate donor unit has been [4]
Mobility of Electric Charge Carriers
As a general rule, the overall efficiency and performance of organic electronics, such as FETs and heterogeneous solar cells, depend on the mobility of the active charge carriers in the system. Self-assembly materials obtained from conjugated gels with π bonds are good options for making organic electronic devices, because the regular molecular arrangement in the gel structure can contribute to the greater mobility of the electric charge carriers in these structures [73, 74]. However, it is very difficult to measure the mobility of charge carriers in gels without destroying their molecular arrangement. Conductive gels with thienylenevinylene base are among the gels in which electric charge carriers have high mobility. Figure 6a shows two examples of these molecular structures (structures 11a and 11b). The large molecules in these gels self-assemble into continuous fibers. The absorption spectra of these materials show that the mobility of the electrons in the mentioned gels is due to the very regular molecular self-arrangement of these materials and the overlap of the band structure of the electron donor and receiver units. Also, if N,N-bis(2,5-di-tert-butylphenyl)-3,4,9,10-perylenedicarboximide (PDI) is used in these gel structures, the mobility of the electrons will increase (Fig. 6b). In fact, it helps to gel more gel on a molecular scale, thereby increasing the mobility of energy carriers. On the other hand, the electron mobility in gel structures is relatively low compared to xerogel structures of the same material (Fig. 6c). It should be noted that if the gel dries at relatively low temperatures, it becomes a very porous solid product called xerogel.
a Molecular structures of thienylenevinylene-based gels (structures 11a and 11b) and optical images of structure 11b in solution (low temperature) and gel (high temperature); b a diagram of the electrical conductivity changes of structure 11b in exchange for the addition of different amounts of PDI duplex; c comparison of the mobility of thin films made of thienylenevinylene-based gels for the case where the film is produced from a solution or xerogel phase [5]
Sensors for Explosives
Photoluminescence is one of the most attractive properties of conductive gels. This is done by absorbing colliding photons with organic matter, stimulating electrons at its HOMO level, and transferring them to the LUMO level. Studies show that if the macromolecules of a conductive gel (which are rich in excited electrons) are exposed to electron-deficient analyses, the gel-stimulated electrons are rapidly transferred to the analytic band structure and photoluminescence properties of these materials they destroy [71, 75]. This interesting phenomenon has recently become the basis for the identification of nitro-aromatic explosives using conductive gels. For example, the presence of TNT explosives in very small amounts (in the range) can be detected using pentafluoroarene functional groups. This is shown in Fig. 7. When these functional groups form a gel-like structure, the macromolecules assume a relatively orderly arrangement and are expected to exhibit photoluminescence properties. Now, if this gel is placed on a special paper in a thin layer, in the presence of sunlight, it will show a special color. If some of the TNT explosives come in contact with the surface of the paper, the photoluminescence property of the gel is rapidly lost, and the rate of drop of this property is directly related to the amount of explosive. The higher the concentration of the explosive, the lower the intensity of the light emitted by the gel, and the presence of an explosive can be detected by the color change. It should be noted that the reason for the suitability of conductive gels for the detection of TNT explosives is the efficient absorption of these materials into the self-assembled structure of the gel molecules and energy absorption by it.
a The chemical structure of conjugated Perfluoropyridine molecules with π bonds that, after molecular self-assembly, are turned into a gel and placed on a piece of paper; b fluorescent microscope image of paper impregnated with conductive gel A exposed to very small amounts of TNT explosives [66]
Applications
Recently, conductive gels have been used in the manufacture of many nanoelectronic devices due to their controllable conductivity. The most important of these applications are organic field-effect transistors (OFETs), organic solar cells, and organic sensors. In the following, the role of these materials in the mentioned applications is described. It should be noted that one of the main applications of conductive gels is the production of hydrogen using water splitting, but since the present article deals with the nanoelectronic applications of conductive gels, this issue will not be discussed [76, 77].
Field-Effect Organic Transistors (OFETs)
Organic semiconductors are preferred over silicon-based semiconductors in many applications, including integrated circuits, sensors, and electronic chips, due to their low weight and flexibility. The relatively regular arrangement of the large molecules in these materials due to the processing of the solution phase can provide continuous conduction paths for the electrons and cavities (percolation network) and increase the efficiency and electrical efficiency of the devices. Since conductive gels are prepared from solution phase and in which large molecule chromophores have the ability of molecular self-assembly, these materials are a very good option for making active materials in field-effect organic transistors (OFETs) [78, 79]. Before discussing the application of conductive gels in OFETs transistors, it is advisable to discuss the general structure of field-effect capacitors. The most common structure of field-effect transistors often consists of a n- and p-type semiconductor junction with two layers of oxide insulating material (such as silicon oxide) and a conductive layer (non-crystalline metal or silicon). Figure 8 shows a schematic of this structure. As can be seen, these transistors consist of three main parts: The main body is a p-type semiconductor that holds two separate n-type semiconductor parts at a certain distance from each other. One type n region is called the source terminal, and the other is called the drain terminal. The area between the source and discharge terminals is covered by a thin, insulating layer of metal oxide, and the conductive layer is placed on top of the oxide layer. The whole area between the two terminals is called the gate terminal. Due to the nature of n-type semiconductors, the concentration of electrons is higher in the areas near the source terminal and discharge, but there is no current between the two regions [80, 81]. Now, if a positive voltage is applied to the gate terminal and the source terminal is connected to ground and the discharge terminal is connected to a positive potential, the negative electrons tend to accumulate in the gate region due to the positive voltage of the gate terminal. If we increase the gate voltage and the positive voltage applied to the discharge source sufficiently, the negative charges accumulated under the metal oxide layer will tend to move toward the gate terminal and the discharge terminal. By doing this, applying a gate voltage and a positive voltage to the discharge terminal causes the electron current to flow from the source terminal to the discharge terminal. In other words, no current of electrons will be established between the two terminals until the gate voltage reaches a critical level. Conversely, if the gate voltage increases exponentially, a path of positive charges in the gate region is created and the intensity of the electron current flux increases. Therefore, the gate terminal acts as a valve for the electron flow tube. It should be noted that if the main body of the field-effect transistor is made of p-type semiconductor, it is called P-FETs and if it is n-type, it is called N-FETs. The same mechanism applies to both types of transistors. In recent years, many attempts have been made to use new materials as the main body or substrate of FETs transistors on which the source and discharge terminals as well as the gate are mounted. Conductive gels are one of these emerging materials. For example, Hong et al. used single nanofibers made of conjugated gels with π bonds as the main body of nanotransistors (Fig. 9). Due to its one-dimensional self-arrangement and chemical structure, this fiber behaves similarly to the p-type semiconductor. The mobility of cavities in this fiber is in the range of 0.48–0.1 cm/s [82]. As shown in Fig. 9, an increase in gate voltage leads to an increase in the current flow from the source terminal to the discharge terminal. In some studies, a thin layer of conductive gels has been used as the main body of OFETs transistors. Figure 5 shows an example of these structures. As can be seen, a field-effect transistor with a downward gate terminal can be produced using a thin layer of conductive gel with a 23 structure. The important point is that the large molecules of this conductive gel have the property of molecular self-assembly, and this leads to high mobility of charge carriers in this thin layer. The structure 23 behaves similarly to the n-type semiconductor, and the majority charge carriers are electrons, the gate voltage is negative and the voltage applied to the discharge terminal is positive. Also, the current–voltage diagrams in Fig. 10 clearly show that as the gate voltage increases and the voltage difference between the source terminal and the discharge increases, the current passing through the transistor increases exponentially. However, the effect of gate voltage on the current density is greater than the discharge terminal voltage.
a Schematic of the three-dimensional structure of field-effect transistors based on p-type semiconductors. b Schematic of the two-dimensional structure of field-effect transistors and the potentials applied to it [57]
a Schematic of the molecular structure of field-effect transistors based on single fibers of 6,2-bis (tiny vinyl) anthracene 2,6-bis (2-thienylvinyl) anthracene, b the SEM image of the transistor a; c and d current–voltage diagrams of transistors based on gate voltage (VGS) and voltage difference between source and discharge terminals (VDS) [58]
a Schematic of the chemical structure of the self-assembling molecule 23; b current–voltage diagram for a field-effect transistor based on structure 23 in terms of gate voltage (VG) and discharge terminal voltage (VSD). The transistor is of type n, and its gate terminal is located below the discharge and source terminals [59]
Organic Solar Cells
Organic solar cells (OSCs) have relatively low efficiencies in converting solar energy into electrical energy, but very low weight, low cost, and flexibility of organic matter relative to inorganic semiconductors are the focus of energy industries. Has attracted. Has attracted Conductive gels, on the other hand, have been considered in comparison with other semiconductor or non-conductive organic materials for several important reasons for making OSCs [83, 84]:
-
(a)
Ease of processing, coating, production of thin films, and production of nanometer fibers.
-
(b)
The possibility of making heterogeneous connections from chromophores giving or receiving large molecules in mesa- or nanodimensions.
-
(c)
Possibility of easy self-assembly of large molecules and engineering of three-dimensional arrangement of electron donor and receiver chromophores to achieve high energy conversion efficiency.
In this section, a sample of organic solar cells based on conductive gels is reviewed. But before entering into this discussion, it is better to introduce the general structure of organic solar cells. Figure 10 schematically shows the layer structure of an organic solar cell. As can be seen, these cells consist of several main parts: (1) glass, (2) a transparent conductive oxide layer (such as indium-tin oxide or ITO), (3) a conductive transparent polymer (such as PEDOT: PSS), (4) an active layer or absorber, (5) a bonding layer, and (6) a conductive metal layer. In general, the conductive metal layer and the transparent metal oxide layer act as “contact points” to connect to the external circuit. A thin layer of transparent conductive polymer such as PEDOT: PSS is usually used as the cavity conductor material directly above the ITO electrode. What plays the most effective role in building an organic solar cell is the “active layer.” Solar cells are divided into several categories in terms of number of layers and electronic architecture (Fig. 10):
-
Organic monolayer solar cells
These solar cells are the simplest type of photovoltaic device in terms of energy storage conversion mechanism. In the structure of these devices, an organic semiconductor is placed between two thin layers of conductive metal. One of these metal layers, such as indium tin oxide (ITO), has a very high working function, and the other layer, such as aluminum or magnesium, has a relatively lower working function (Fig. 10a). The mechanism of storage of electric charge is such that due to the collision of the landing photon, a large number of exactions (electron–hole pair) are formed in the band structure of the organic semiconductor layer. These electrons begin to move due to the electric field created between the two metal layers due to the difference in their working function [84]. They flow toward the positive metal electrode and the cavity toward the negative electrode. The band structure of this type of solar cell and the path of electrons and holes are shown in Fig. 10a.
-
Organic bilayer solar cells
This type of solar cell is made of two separate organic layers with completely different electronegativity between conductive metal layers. This difference in electronegativity leads to the creation of an electrostatic field between two metal layers. The semiconductor layer that has a high electronegativity tends to absorb more electrons and the other organic layer tends to give electrons. Therefore, when a landing photon strikes these two semiconductor layers, it leads to the formation of an electron–hole pair at the LUMO level of the band structure of the two materials, and the electrons and holes due to the electrostatic field created between the two layers begin to separate and move. They do it in the opposite direction. In other words, the layer with high electronegativity acts as the electron acceptor and repulse of the cavity, and the layer with lower electronegativity acts as the electron giver and the absorber of the cavity. In this way, the exactions are separated from each other and placed in holes in the outer circuit by holes and electrons through metal layers. The band structure of this group of solar cells and the path of electron–hole motion are shown in Fig. 11.
-
Organic solar cells with mass heterogeneous connections
In organic solar cells, two metal layers (mostly aluminum and ITO) are used as the two points of contact for the external circuit. Also, conductive gels are often used as the organic layer between these two conductive metal layers. This layer is also called the active layer. Recently, some researchers have used organic gels based on conductive polymers as the active layer in organic solar cells [85]. Figure 12 shows the chemical structure of two types of polymer molecules (structures 26a and 26b). These two molecules are similar in molecular structure, but one of them has a CN functional group. The use of these materials as an active layer in organic solar cells has been shown to improve the energy storage efficiency of these systems by 1.76%. This efficiency is also increased if heat annealing is used to convert the solution containing these molecules into gel structures in which molecules 26a and 26b are self-arranged. This shows how much the gelling of solutions containing conjugated polymers with π bonds can affect the self-assembly of molecules and energy storage efficiency. Figure 12 shows the increase in efficiency of generated solar cells in the form of stored current density graphs. Molecular structure 29 in Fig. 13 is another example of the application of conductive gels in the fabrication of solar organic cells as the active layer. These large molecules can affect energy conversion efficiency, depending on the number of repetitive units and the degree to which they are self-assembled. As can be seen in Fig. 13, gelling and self-assembly of large molecules significantly increase the slope of changes in the energy conversion efficiency diagram. Efforts to replace various types of conductive gels in the main body of organic solar cells continue today.
a Schematic of the general structure of organic solar cells; b the TEM image of the said solar cell. This solar cell has several single layers: glass or SiO2, indium tin oxide or ITO, clear polymers and the active layer PEDOT: PSS and MDMO-PPV: PCBM, the intermediate layer LiF, and the conductive metal layer or aluminum [72]
Chemical structures of conductive organic gels (structures 26a and 26b) and energy conversion efficiency diagrams in annealed and non-annealed samples. Annealing only results in molecular self-assembly of conjugated polymers with π bonds [86]
Chemical structures of barbarity-functionalized oligothiophene derivatives (structures 29a, 29b, and 29c) and diagrams of energy conversion efficiency in annealed thin films of these molecules at 80 °C. Annealing causes molecular self-assembly and the formation of coarse particles forming a conductive gel [73]
Conclusion
-
a.
In the organic nanoelectronics industry, in addition to organic charge transfer complexes and conductive polymers, conjugated gels with π bonds are also used. These materials are large molecules that, similar to organic charge transfer complexes, have electron donor and receptor centers and, like conductive polymers, are composed of conjugated molecules with π bonds. These large molecular units in gel structures are placed next to each other with non-covalent bonds, increasing the viscosity of the system. One of the main features of conductive gels is the ability to change the optical behavior of these materials by controlling chromophores, viscosity, arrangement of molecular arrays, and doping with other molecules. These materials can also be used in photon upconversion technology. Conductive gels are now used in the manufacture of solar cells to convert solar energy into electricity.
-
b.
In the present paper, the nanoelectronic applications of conductive gels were reviewed. The most important of these applications are sensors for detecting explosives, thin-film organic solar cells, and field-effect nanotransistors. As mentioned, conductive gels can be used as non-intrinsic semiconductors due to the self-arrangement of large molecules and can be used as the active layer in organic solar cells or the main body of semiconductors in field-effect transistors. The basis of these materials is the excitation of HOMO level electrons and their transfer to the LUMO level. The efficiency and performance of this group of materials are strongly related to the kinetics of the process of excitation and transfer of charge carriers due to the collision of landing photons. Conductivity can significantly increase the conductivity of conductive gels. The most important method is to dope or add a specific functional group to the molecular arrays of these materials. More details of the mentioned applications are discussed in the present article.
Availability of data and materials
Not applicable.
Change history
14 November 2022
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1186/s11671-022-03746-9
Abbreviations
- HOMO:
-
Highest occupied molecular orbital
- LUMO:
-
Lowest unoccupied molecular orbital
- TTF:
-
Tetrathiafulvalene
References
Haedler AT, Kreger K, Issac A, Wittmann B, Kivala M, Hammer N, Köhler J, Schmidt H-W, Hildner R (2015) Long-range energy transport in single supramolecular anofibers at room temperature. Nature 523(7559):196–199
Haedler AT, Beyer SR, Hammer N, Hildner R, Kivala M, Köhler J, Schmidt H-W (2014) Synthesis photophysical properties of multichromophoric carbonyl-bridged triarylamines. Chem Eur J 20(37):11708–11718
Ogawa T, Yanai N, Monguzzi A, Kimizuka N (2015) Highly efficient photon upcon in self-assembled light-harvesting molecular systems. Sci Rep 5:1–9
Cheriya RT, Mallia AR, Hariharan M (2014) Light harvesting vesicular donor–acceptor scaffolds the rate of charge recombination in the presence of an electron donor. Energy Environ Sci 7(5):1661–1669
Prasanthkumar S, Saeki A, Seki S, Ajayaghosh A (2010) Solution phase epitaxial self-assemblyhigh charge-carrier mobility nanofibers of semiconducting molecular gelators. J Am Chem Soc 132(26):8866–8867
Ghosh S, Praveen VK, Ajayaghosh A (2016) The chemistryapplications of π-gels. Annu Rev Mater Res 46:235–262
He Q, Liu J, Liang J, Liu X, Li W, Liu Z, Ding Z, Tuo D (2018) Towards improvements for penetrating the blood-brain barrier—recent progress from a material and pharmaceutical perspective. Cells 7:24. https://doi.org/10.3390/cells7040024
Zhou T, Xiao X, Li G (2012) Microwave accelerated selective soxhlet extraction for the determination of organophosphorus and carbamate pesticides in ginseng with gas chromatography/mass spectrometry. Anal Chem 84(13):5816–5822. https://doi.org/10.1021/ac301274r
Wu Y, Deng P, Tian Y et al (2020) Simultaneous and sensitive determination of ascorbic acid, dopamine and uric acid via an electrochemical sensor based on PVP-graphene composite. J Nanobiotechnol 18:112. https://doi.org/10.1186/s12951-020-00672-9
Li GL, Zhong P, Ye YB, Wan X, Cai ZT, Yang SH, Xia YH, Li Q, Liu J, He QG (2019) A highly sensitive and stable dopamine sensor using shuttle-like alpha-Fe2O3 nanoparticles/electro-reduced graphene oxide composites. J Electrochem Soc 166(15):B1552–B1561
Tian Y, Deng P, Wu Y, Liu J, Li J, Li G, He Q (2020) High sensitive voltammetric sensor for nanomolarity vanillin detection in food samples via manganese dioxide nanowires hybridized electrode. Microchem J 157:104885
Wu Z, Yang X, Wu J (2021) Conductive hydrogel- and organohydrogel-based stretchable sensors. ACS Appl Mater Interfaces 13(2):2128–2144. https://doi.org/10.1021/acsami.0c21841
Zhang X, Tang Y, Zhang F, Lee C (2016) A novel aluminum-graphite dual-ion battery. Adv Energy Mater 6(11):1502588. https://doi.org/10.1002/aenm.201502588
Wang M, Jiang C, Zhang S, Song X, Tang Y, Cheng H (2018) Reversible calcium alloying enables a practical room-temperature rechargeable calcium-ion battery with a high discharge voltage. Nat Chem 10(6):667–672. https://doi.org/10.1038/s41557-018-0045-4
Mu S, Liu Q, Kidkhunthod P, Zhou X, Wang W, Tang Y (2020) Molecular grafting towards high-fraction active nanodots implanted in N-doped carbon for sodium dual-ion batteries. Natl Sci Rev. https://doi.org/10.1093/nsr/nwaa178
Zhu W, Deng M, Chen D, Zhang Z, Chai W, Chen D, Xi H, Zhang J, Zhang C, Hao Y (2020) Dual-phase CsPbCl3–Cs4PbCl6 perovskite films for self-powered, visible-blind UV photodetectors with fast response. ACS Appl Mater Interfaces 12(29):32961–32969. https://doi.org/10.1021/acsami.0c09910
Liu Y, Zhang Q, Yuan H, Luo K, Li J, Hu W, Bazaka K (2021) Comparative study of photocatalysis and gas sensing of ZnO/Ag nanocomposites synthesized by one- and two-step polymer-network gel processes. J Alloys Compd 868:158723. https://doi.org/10.1016/j.jallcom.2021.158723
Liu W, Zheng Y, Wang Z, Wang Z, Yang J, Chen M, Qi M, Ur Rehman S, Shum PP, Zhu L, Wei L (2021) Ultrasensitive exhaled breath sensors based on anti-resonant hollow core fiber with in situ grown ZnO–Bi2O3 nanosheets. Adv Mater Interfaces. https://doi.org/10.1002/admi.202001978
Zhang Z, Feng L, Liu H, Wang L, Wang S, Tang Z (2021) Mo6+–P5+ co-doped Li2ZnTi3O8 anode for Li-storage in a wide temperature range and applications in LiNi0.5Mn1.5O4/Li2ZnTi3O8 full cells. Inorg Chem Front 9(1):35–43. https://doi.org/10.1039/D1QI01077H
Zhang Y, Li C, Jia D, Zhang D, Zhang X (2015) Experimental evaluation of the lubrication performance of MoS2/CNT nanofluid for minimal quantity lubrication in Ni-based alloy grinding. Int J Mach Tools Manuf 99:19–33
Zhang Y, Li C, Ji H, Yang X, Yang M, Jia D, Zhang X, Li R, Wang J (2017) Analysis of grinding mechanics and improved predictive force model based on material-removal and plastic-stacking mechanisms. Int J Mach Tools Manuf 122:81–97
Yang M, Li C, Zhang Y, Jia D, Zhang X, Hou Y, Li R, Wang J (2017) Maximum undeformed equivalent chip thickness for ductile-brittle transition of zirconia ceramics under different lubrication conditions. Int J Mach Tools Manuf 122:55–65
Li B, Li C, Zhang Y, Wang Y, Jia D, Yang M, Zhang N, Wu Q, Han Z, Sun K (2017) Heat transfer performance of MQL grinding with different nanofluids for Ni-based alloys using vegetable oil. J Clean Prod 154:1–11
Guo S, Li C, Zhang Y, Wang Y, Li B, Yang M, Zhang X, Liu G (2017) Experimental evaluation of the lubrication performance of mixtures of castor oil with other vegetable oils in MQL grinding of nickel-based alloy. J Clean Prod 140(3):1060–1076
Wang Y, Li C, Zhang Y, Li B, Yang M, Zhang X, Guo S, Liu G (2016) Experimental evaluation of the lubrication properties of the wheel/workpiece interface in MQL grinding with different nanofluids. Tribol Int 99(1):198–210
Salimi M, Pirouzfar V, Kianfar E (2017) Enhanced gas transport properties in silica nanoparticle filler-polystyrene nanocomposite membranes. Colloid Polym Sci 295:215–226. https://doi.org/10.1007/s00396-016-3998-0
Kianfar E (2018) Synthesis and characterization of AlPO4/ZSM-5 catalyst for methanol conversion to dimethyl ether. Russ J Appl Chem 91:1711–1720. https://doi.org/10.1134/S1070427218100208
Kianfar E (2019) Ethylene to propylene conversion over Ni-W/ZSM-5 catalyst. Russ J Appl Chem 92:1094–1101. https://doi.org/10.1134/S1070427219080068
Kianfar E (2019) Ethylene to propylene over zeolite ZSM-5: improved catalyst performance by treatment with CuO. Russ J Appl Chem 92:933–939. https://doi.org/10.1134/S1070427219070085
Kianfar E, Shirshahi M, Kianfar F et al (2018) Simultaneous prediction of the density, viscosity and electrical conductivity of pyridinium-based hydrophobic ionic liquids using artificial neural network. SILICON 10:2617–2625. https://doi.org/10.1007/s12633-018-9798-z
Babu SS, Praveen VK, Ajayaghosh A (2014) Functional π-gelatorstheir applications. Chem Rev 114(4):1973–2129
Salimi M, Pirouzfar V, Kianfar E (2017) Novel nanocomposite membranes prepared with PVC/ABS and silica nanoparticles for C2H6/CH4 separation. Polym Sci Ser A 59:566–574. https://doi.org/10.1134/S0965545X17040071
Kianfar F, Kianfar E (2019) Synthesis of isophthalic acid/aluminum nitrate thin film nanocomposite membrane for hard water softening. J Inorg Organomet Polym 29:2176–2185. https://doi.org/10.1007/s10904-019-01177-1
Kianfar E, Azimikia R, Faghih SM (2020) Simple and strong dative attachment of α-diimine nickel (II) catalysts on supports for ethylene polymerization with controlled morphology. Catal Lett 150:2322–2330. https://doi.org/10.1007/s10562-020-03116-z
Kianfar E (2019) Nanozeolites: synthesized, properties, applications. J Sol Gel Sci Technol 91:415–429. https://doi.org/10.1007/s10971-019-05012-4
Liu H, Kianfar E (2020) Investigation the synthesis of nano-SAPO-34 catalyst prepared by different templates for MTO process. Catal Lett. https://doi.org/10.1007/s10562-020-03333-6
Puigmartí-Luis J, Pérez del Pino Á, Laukhina E, Esquena J, Laukhin V, Rovira C, Vidal-Gancedo J et al (2008) Shaping supramolecular nanofibers with nanoparticles forming complementary hydrogen bonds. Angew Chem Int Ed 47(10):1861–1865
Kianfar E, Salimi M, Hajimirzaee S, Koohestani B (2018) Methanol to gasoline conversion over CuO/ZSM-5 catalyst synthesized using sonochemistry method. Int J Chem React Eng 17
Kianfar E, Salimi M, Pirouzfar V, Koohestani B (2018) Synthesis of modified catalyst and stabilization of CuO/NH4-ZSM-5 for conversion of methanol to gasoline. Int J Appl Ceram Technol 15:734–741. https://doi.org/10.1111/ijac.12830
Kianfar E, Salimi M, Pirouzfar V, Koohestani B (2018) Synthesis and modification of zeolite ZSM-5 catalyst with solutions of calcium carbonate (CaCO3) and sodium carbonate (Na2CO3) for methanol to gasoline conversion. Int J Chem React Eng 16(7):20170229. https://doi.org/10.1515/ijcre-2017-0229
Ehsan K (2019) Comparison and assessment of zeolite catalysts performance dimethyl ether and light olefins production through methanol: a review. Rev Inorg Chem 39:157–177
Ehsan K, Salimi M (2020) A review on the production of light olefins from hydrocarbons cracking and methanol conversion. In: Taylor JC (ed) Advances in chemistry research, vol 59. Chapter: 1. Nova Science Publishers, New York
Ehsan K, Razavi A (2020) Zeolite catalyst based selective for the process MTG: a review. In: Mahler A (ed) Zeolites: advances in research and applications. Chapter: 8. Nova Science Publishers, New York
Ehsan K (2020) Zeolites: properties, applications, modification and selectivity. In: Mahler A (ed) Zeolites: advances in research and applications, Chapter: 1. Nova Science Publishers, New York
Kianfar E, Hajimirzaee S, Musavian SS, Mehr AS (2020) Zeolite-based catalysts for methanol to gasoline process: a review. Microchem J 156:104822
Shi Y, Yu G (2016) Designing hierarchically nanostructured conductive polymer gels for electrochemical energy storage and conversion. Chem Mater 28:2466–2477
Kianfar E, Baghernejad M, Rahimdashti Y (2015) Study synthesis of vanadium oxide nanotubes with two template hexadecylamin and hexylamine. Biol Forum 7:1671–1685
Ehsan K (2020) Synthesizing of vanadium oxide nanotubes using hydrothermal and ultrasonic method. Lambert Academic Publishing, Sunnyvale, pp 1–80
Shi Y, Peng L, Ding Y, Zhao Y, Yu G (2015) Nanostructured conductive polymers for advanced energy storage. Chem Soc Rev 44:6684–6696. https://doi.org/10.1039/C5CS00362H
Kianfar E, Pirouzfar V, Sakhaeinia H (2017) An experimental study on absorption/stripping CO2 using Mono-ethanol amine hollow fiber membrane contactor. J Taiwan Inst Chem Eng 80:954–962
Kianfar E, Viet C (2021) Polymeric membranes on base of PolyMethyl methacrylate for air separation: a review. J Mater Res Technol 10:1437–1461
Faravar P, Zarei Z, Monjezi MG (2020) Modeling and simulation absorption of CO2 using hollow fiber membranes (HFM) with mono-ethanol amine with computational fluid dynamics. J Environ Chem Eng 8(4):103946
Yang Z, Zhang L, Zhou Y, Wang H, Wen L, Kianfar E (2020) Investigation of effective parameters on SAPO-34 Nano catalyst the methanol-to-olefin conversion process: a review. Rev Inorg Chem 40(3):91–105
Gao C, Liao J, Jingqiong Lu, Ma J, Kianfar E (2020) The effect of nanoparticles on gas permeability with polyimide membranes and network hybrid membranes: a review. Rev Inorg Chem. https://doi.org/10.1515/revic-2020-0007
To JWF, Chen Z, Yao H, He J, Kim K, Chou H-H, Pan L, Wilcox J, Cui Y, Bao Z (2015) Ultrahigh surface area three-dimensional porous graphitic carbon from conjugated polymeric molecular framework. ACS Cent Sci 1:68–76. https://doi.org/10.1021/acscentsci.5b00149
Stone DA, Tayi AS, Goldberger JE, Palmer LC, Stupp SI (2011) Self-assemblyconductivity of hydrogen-bonded oligothiophene nanofiber networks. Chem Commun 47(20):5702–5704
http://olli.informatik.uni-oldenburg.de/weTEiS/weteis/transistor1.htm
Hong J-P, Um M-C, Nam S-R, Hong J-I, Lee S (2009) Oranic single-nanofiber transistors organogels. Chem Commun 3:310–312
Tsai W-W, Tevis ID, Tayi AS, Cui H, Stupp SI (2010) Semiconducting nanowires hairpin-shaped self-assembling sexithiophenes. J Phys Chem B 114(45):14778–14786
Kianfar E, Salimi M, Koohestani B (2020) Zeolite CATALYST: a review on the production of light olefins. Lambert Academic Publishing, Sunnyvale, pp 1–116
Ehsan K (2020) Investigation on catalysts of “Methanol to light Olefins.” Lambert Academic Publishing, Sunnyvale, pp 1–168
Kianfar E (2020) Application of nanotechnology in enhanced recovery oil and gas importance & applications of nanotechnology, chapter 3, vol 5. MedDocs Publishers, Reno, pp 16–21
Kianfar E (2020) Catalytic properties of nanomaterials and factors affecting it. Importance & applications of nanotechnology, chapter 4, vol 5. MedDocs Publishers, Reno, pp 22–25
Shi Y, Peng L, Yu G (2015) Nanostructured conducting polymer hydrogels for energy storage applications. Nanoscale 7:12796–12806. https://doi.org/10.1039/C5NR03403E
Zhao Y, Liu B, Pan L, Yu G (2013) 3D nanostructured conductive polymer hydrogels for high-performance electrochemical devices. Energy Environ Sci 6:2856–2870. https://doi.org/10.1039/c3ee40997j
Kartha KK, Babu SS, Srinivasan S, Ajayaghosh A (2012) Attogram sensing of trinitrotoluene with a self-assembled molecular gelator. J Am Chem Soc 134(10):4834–4841
Kianfar E (2020) Introducing the application of nanotechnology in lithium-ion battery importance & applications of nanotechnology, chapter 4, vol 4. MedDocs Publishers, Reno, pp 1–7
Ehsan K, Mazaheri H (2020) Synthesis of nanocomposite (CAU-10-H) thin-film nanocomposite (TFN) membrane for removal of color from the water. Fine Chem Eng 1:83–91
Ehsan K, Salimi M, Koohestani B (2020) Methanol to gasoline conversion over CuO/ZSM-5 catalyst synthesized and influence of water on conversion. Fine Chem Eng 1:75–82
Kianfar E (2020) An experimental study PVDF and PSF hollow fiber membranes for chemical absorption carbon dioxide. Fine Chem Eng 1:92–103
Zhang C et al (2017) Broadband metamaterial for optical transparency and microwave absorption. Appl Phys Lett 110(14):143511
Dennler G, Scharber MC, Brabec CJ (2009) Polymer-fullerene bulk-heterojunction solar cells. Adv Mater 21(13):1323–1338
Kim BG, Jeong EJ, Park HJ, Bilby D, Guo LJ, Kim J (2011) Effect of polymer aggregation on the open circuit voltage in organic photovoltaic cells: aggregation-induced conjugated polymer gelits application for preventing open circuit voltage drop. ACS Appl Mater Interfaces 3(3):674–680
Yagai S, Suzuki M, Lin X, Gushiken M, Noguchi T, Karatsu T, Kitamura A et al (2014) Supramolecular engineering of oligothiophene nanorods without insulators: hierarchical association of rosettesphotovoltaic properties. Chem Eur J 20(49):16128–16137
Zhou Y, Zhang M, Guo Z, Miao L, Han ST, Wang Z, Zhang X, Zhang H, Peng Z (2017) Recent advances in black phosphorus-based photonics, electronics, sensors and energy devices. Mater Horiz 4(6):997–1019
Lee IG, Yoon SH, Lee JS, Hong IP (2016) Design of wideband radar absorbing material with improved optical transmittance by using printed metal-mesh. Electron Lett 52(7):555–557
Yakovlev AV, Milichko VA, Vinogradov VV, Vinogradov AV (2016) Inkjet color printing by interference nanostructures. ACS Nano 10(3):3078–3086
Qu B, Zhu C, Li C, Zhang X, Chen Y (2016) Coupling hollow Fe3O4-Fe nanoparticles with graphene sheets for high-performance electromagnetic wave absorbing material. ACS Appl Mater Interfaces 8(6):3730–3735. https://doi.org/10.1021/acsami.5b12789
Li Y, Li W, Wang Y, Cao J, Guan J (2018) Refractory metamaterial microwave absorber with strong absorption insensitive to temperature. Adv Opt Mater 6:1800691. https://doi.org/10.1002/adom.201800691
Gao K, Jo SB, Shi X, Nian L, Zhang M, Kan Y, Lin F, Kan B, Xu B, Rong Q, Shui L (2019) Over 12% efficiency nonfullerene all-small-molecule organic solar cells with sequentially evolved multilength scale morphologies. Adv Mater 31(12):1807842
Yu Z, Jiao S, Li S, Chen X, Song W-L, Teng T, Jiguo Tu, Chen H-S, Zhang G, Fang D-N (2019) Flexible stable solid-state Al-ion batteries. Adv Funct Mater 29(1):1806799
Wang Y, Zhang KL, Zhang BX, Ma CJ, Song WL, Hou ZL, Chen M (2018) Smart mechano-hydro-dielectric coupled hybrid sponges for multifunctional sensors. Sens Actuators B Chem 270:239–246
Xu H, Yin X, Li M, Ye F, Han M, Hou Z, Li X, Zhang L, Cheng L (2018) Mesoporous carbon hollow microspheres with red blood cell like morphology for efficient microwave absorption at elevated temperature. Carbon 132:343–351
Chen H, Ma W, Huang Z, Zhang Y, Huang Y, Chen Y (2019) Graphene-based materials toward microwave and terahertz absorbing stealth technologies. Adv Opt Mater 7(8):1801318
Xiang Y, Chen D (2007) Preparation of a novel pH-responsive silver nanoparticle/poly(HEMA-PEGMA-MAA) composite hydrogel. Eur Polym J 43:4178–4187
McGehee MD, Topinka MA (2006) Solar cells: pictures the blended zone. Nat Mater 5(9):675–676
Devaki SJ, Narayanan RK, Sarojam S (2014) Electrically conducting silver nanoparticle-polyacrylic acid hydrogel by in situ reduction and polymerization approach. Mater Lett 116:135–138
Fantino E, Chiappone A, Roppolo I et al (2016) 3D printing of conductive complex structures with in situ generation of silver nanoparticles. Adv Mater 28:3712–3717
Hyun DC, Park M, Park C et al (2011) Ordered zigzag stripes of polymer gel/metal nanoparticle composites for highly stretchable conductive electrodes. Adv Mater 23:2946–2950
Janovák L, Dékány I (2010) Optical properties and electric conductivity of gold nanoparticle-containing, hydrogel-based thin layer composite films obtained by photopolymerization. Appl Surf Sci 256:2809–2817
Pardo-Yissar V, Gabai R, Shipway AN, Bourenko T, Willner I (2001) Gold nanoparticle/hydrogel composites with solvent-switchable electronic properties. Adv Mater 13:1320–1323
Zhao X, Ding X, Deng Z, Zheng Z, Peng Y, Long X (2005) Thermoswitchable electronic properties of a gold nanoparticle/hydrogel composite. Macromol Rapid Commun 26:1784–1787
Baei P, Jalili-Firoozinezhad S, Rajabi-Zeleti S, Tafazzoli-Shadpour M, Baharvand H, Aghdami N (2016) Electrically conductive gold nanoparticle-chitosan thermosensitive hydrogels for cardiac tissue engineering. Mater Sci Eng C 63:131–141
Navaei A, Saini H, Christenson W, Sullivan RT, Ros R, Nikkhah M (2016) Gold nanorod-incorporated gelatin-based conductive hydrogels for engineering cardiac tissue constructs. Acta Biomater 41:133–146
Wei Q, Luo Y, Zhang C, Fan L, Chen Y (2008) Assembly of Cu nanoparticles in a polyacrylamide grafted poly(vinyl alcohol) copolymer matrix and vapor-induced response. Sens Actuators B Chem 134:49–56
Ehsan K, Mafi S (2020) Ionic liquids: properties, application, and synthesis. Fine Chem Eng 2:22–31
Faghih SM, Kianfar E (2018) Modeling of fluid bed reactor of ethylene dichloride production in Abadan Petrochemical based on three-phase hydrodynamic model. Int J Chem React Eng 16:1–14
Ehsan K, Mazaheri H (2020) Methanol to gasoline: a sustainable transport fuel. In: Taylor JC (ed) Advances in chemistry research, chapter: 4. Nova Science Publishers, Hauppauge, p 66
Han L, Liu K, Wang M et al (2018) Mussel-inspired adhesive and conductive hydrogel with long-lasting moisture and extreme temperature tolerance. Adv Funct Mater 28:1704195
Liang Y, Zhao X, Hu T, Han Y, Guo B (2019) Mussel-inspired, antibacterial, conductive, antioxidant, injectable composite hydrogel wound dressing to promote the regeneration of infected skin. J Colloid Interface Sci 556:514–528
Cai G, Wang J, Qian K, Chen J, Li S, Lee PS (2017) Extremely stretchable strain sensors based on conductive self-healing dynamic cross-links hydrogels for human-motion detection. Adv Sci 4:1600190
Mottet L, Le Cornec D, Noël JM et al (2018) A conductive hydrogel based on alginate and carbon nanotubes for probing microbial electroactivity. Soft Matter 14:1434–1441
Deng Z, Hu T, Lei Q, He J, Ma PX, Guo B (2019) Stimuli-responsive conductive nanocomposite hydrogels with high stretchability, self-healing, adhesiveness, and 3D printability for human motion sensing. ACS Appl Mater Interfaces 11:6796–6808
Xu Y, Lin Z, Huang X, Wang Y, Huang Y, Duan X (2013) Functionalized graphene hydrogel-based high-performance supercapacitors. Adv Mater 25:5779–5784
Liu Q, Zhang M, Huang L et al (2015) High-quality graphene ribbons prepared from graphene oxide hydrogels and their application for strain sensors. ACS Nano 9:12320–12326
Xiao X, Wu G, Zhou H, Qian K, Hu J (2017) Preparation and property evaluation of conductive hydrogel using poly(vinyl alcohol)/polyethylene glycol/graphene oxide for human electrocardiogram acquisition. Polymers 9:259
Alam A, Meng Q, Shi G et al (2016) Electrically conductive, mechanically robust, pH-sensitive graphene/polymer composite hydrogels. Compos Sci Technol 127:119–126
Annabi N, Shin SR, Tamayol A et al (2016) Highly elastic and conductive human-based protein hybrid hydrogels. Adv Mater 28:40–49
Han L, Lu X, Wang M et al (2017) A mussel-inspired conductive, self-adhesive, and self-healable tough hydrogel as cell stimulators and implantable bioelectronics. Small 13:1601916
Liang Y, Zhao X, Hu T et al (2019) Adhesive hemostatic conducting injectable composite hydrogels with sustained drug release and photothermal antibacterial activity to promote full-thickness skin regeneration during wound healing. Small 15:1900046
Jing X, Mi HY, Napiwocki BN, Peng XF, Turng LS (2017) Mussel-inspired electroactive chitosan/graphene oxide composite hydrogel with rapid self-healing and recovery behavior for tissue engineering. Carbon 125:557–570
Qiu L, Liu D, Wang Y et al (2014) Mechanically robust, electrically conductive and stimuli-responsive binary network hydrogels enabled by superelastic graphene aerogels. Adv Mater 26:3333–3337
Wang Y, Huang F, Chen X et al (2018) Stretchable, conductive, and self-healing hydrogel with super metal adhesion. Chem Mater 30:4289–4297
Mano N, Yoo JE, Tarver J, Loo YL, Heller A (2007) An electron-conducting cross-linked polyaniline-based redox hydrogel, formed in one step at pH 7.2, wires glucose oxidase. J Am Chem Soc 129:7006–7007
Guarino V, Alvarez-Perez MA, Borriello A, Napolitano T, Ambrosio L (2013) Conductive PANi/PEGDA macroporous hydrogels for nerve regeneration. Adv Healthc Mater 2:218–227
Dong R, Zhao X, Guo B, Ma PX (2016) Self-healing conductive injectable hydrogels with antibacterial activity as cell delivery carrier for cardiac cell therapy. ACS Appl Mater Interfaces 8:17138–17150
Zhao X, Wu H, Guo B, Dong R, Qiu Y, Ma PX (2017) Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials 122:34–47
Qu J, Zhao X, Ma PX, Guo B (2018) Injectable antibacterial conductive hydrogels with dual response to an electric field and pH for localized “smart” drug release. Acta Biomater 72:55–69
Guo B, Qu J, Zhao X, Zhang M (2019) Degradable conductive self-healing hydrogels based on dextran-graft-tetraaniline and N-carboxyethyl chitosan as injectable carriers for myoblast cell therapy and muscle regeneration. Acta Biomater 84:180–193
Li W, Gao F, Wang X, Zhang N, Ma M (2016) Strong and robust polyaniline-based supramolecular hydrogels for flexible supercapacitors. Angew Chem Int Ed 55:9196–9201
Pan L, Yu G, Zhai D et al (2012) Hierarchical nanostructured conducting polymer hydrogel with high electrochemical activity. PNAS 109:9287–9292
Guo H, He W, Lu Y, Zhang X (2015) Self-crosslinked polyaniline hydrogel electrodes for electrochemical energy storage. Carbon 92:133–141
Chakraborty P, Guterman T, Adadi N et al (2019) A self-healing, all-organic, conducting, composite peptide hydrogel as pressure sensor and electrogenic cell soft substrate. ACS Nano 13:163–175
Shi Y, Ma C, Peng L, Yu G (2015) Conductive, “smart” hybrid hydrogels with PNIPAM and nanostructured conductive polymers. Adv Funct Mater 25:1219–1225
Deng Z, Guo Y, Ma PX, Guo B (2018) Rapid thermal responsive conductive hybrid cryogels with shape memory properties, photothermal properties and pressure dependent conductivity. J Colloid Interface Sci 526:281–294
Chen J, Peng Q, Thundat T, Zeng H (2019) Stretchable, injectable, and self-healing conductive hydrogel enabled by multiple hydrogen bonding toward wearable electronics. Chem Mater 31:4553–4563
Wang Z, Zhou H, Lai J et al (2018) Extremely stretchable and electrically conductive hydrogels with dually synergistic networks for wearable strain sensors. J Mater Chem C 6:9200–9207
Hur J, Im K, Kim SW et al (2014) Polypyrrole/agarose-based electronically conductive and reversibly restorable hydrogel. ACS Nano 8:10066–10076
Shi Y, Pan L, Liu B et al (2014) Nanostructured conductive polypyrrole hydrogels as high-performance, flexible supercapacitor electrodes. J Mater Chem A 2:6086–6091
Han L, Yan L, Wang M et al (2018) Transparent, adhesive, and conductive hydrogel for soft bioelectronics based on light-transmitting polydopamine-doped polypyrrole nanofibrils. Chem Mater 30:5561–5572
Gan D, Han L, Wang M et al (2018) Conductive and tough hydrogels based on biopolymer molecular templates for controlling in situ formation of polypyrrole nanorods. ACS Appl Mater Interfaces 10:36218–36228
Rong Q, Lei W, Chen L, Yin Y, Zhou J, Liu M (2017) Anti-freezing, conductive self-healing organohydrogels with stable strain-sensitivity at subzero temperatures. Angew Chem Int Ed 56:14159–14163
Gotovtsev PM, Badranova GU, Zubavichus YV et al (2019) Electroconductive PEDOT:PSS-based hydrogel prepared by freezing-thawing method. Heliyon 5:e02498
Mawad D, Artzy-Schnirman A, Tonkin J et al (2016) Electroconductive hydrogel based on functional poly(ethylenedioxy thiophene). Chem Mater 28:6080–6088
Lu B, Yuk H, Lin S et al (2019) Pure PEDOT:PSS hydrogels. Nat Commun 10:1043
Kianfar (2020) A comparison and assessment on performance of zeolite catalyst based selective for the process methanol to gasoline: a review. In: Advances in chemistry research, chapter 2. Nova Science Publishers, New York, p 63
Kianfar E, Salimi M, Kianfar F et al (2019) CO2/N2 separation using polyvinyl chloride iso-phthalic acid/aluminium nitrate nanocomposite membrane. Macromol Res 27:83–89. https://doi.org/10.1007/s13233-019-7009-4
Ehsan K (2020) Synthesis of characterization Nanoparticles isophthalic acid/aluminum nitrate (CAU-10-H) using method hydrothermal. Advances in chemistry research. Nova Science Publishers, New York
Ehsan K (2020) CO2 capture with ionic liquids: a review. Advances in chemistry research, vol 67. Nova Science Publishers, New York
Ehsan K (2020) Enhanced light olefins production via methanol dehydration over promoted SAPO-34. Advances in chemistry research, chapter: 4, Nova Science Publishers, New York, p 63
Ehsan K (2020) Gas hydrate: applications, structure, formation, separation processes, thermodynamics. In: Taylor JC (ed) Advances in Chemistry research, chapter: 8. Nova Science Publishers, New York, p 62
Kianfar M, Kianfar F, Kianfar E (2016) The effect of nano-composites on the mechanic and morphological characteristics of NBR/PA6 blends. Am J Oil Chem Technol 4(1):29–44
Farshad K, Moghadam SRM, Kianfar E (2015) Energy optimization of ilam gas refinery unit 100 by using HYSYS refinery software. Indian J Sci Technol 8(S9):431–436
Kianfar E (2015) Production and identification of vanadium oxide nanotubes. Indian J Sci Technol 8(S9):455–464
Farshad K, Moghadam SRM, Kianfar E (2015) Synthesis of spiro pyran by using silica-bonded N-propyldiethylenetriamine as recyclable basic catalyst. Indian J Sci Technol 8(11):68669
Kianfar E (2019) Recent advances in synthesis, properties, and applications of vanadium oxide nanotube. Microchem J 145:966–978
Hajimirzaee S, Soleimani Mehr A, Kianfar E (2020) Modified ZSM-5 zeolite for conversion of LPG to aromatics. Polycycl Arom Compd. https://doi.org/10.1080/10406638.2020.1833048
Li L, Wang Y, Pan L et al (2015) A nanostructured conductive hydrogels-based biosensor platform for human metabolite detection. Nano Lett 15:1146–1151
Das S, Chakraborty P, Mondal S, Shit A, Nandi AK (2016) Enhancement of energy storage and photoresponse properties of folic acid-polyaniline hybrid hydrogel by in situ growth of Ag nanoparticles. ACS Appl Mater Interfaces 8:28055–28067
Li P, Jin Z, Peng L et al (2018) Stretchable all-gel-state fiber-shaped supercapacitors enabled by macromolecularly interconnected 3D graphene/nanostructured conductive polymer hydrogels. Adv Mater 30:1800124
Guo Y, Zheng K, Wan P (2018) A flexible stretchable hydrogel electrolyte for healable all-in-one configured supercapacitors. Small 14:1704497
Rong Q, Han H, Feng F, Ma Z (2015) Network nanostructured polypyrrole hydrogel/Au composites as enhanced electrochemical biosensing platform. Sci Rep 5:11440. https://doi.org/10.1038/srep11440
Yang J, Wang X, Li B et al (2017) Novel iron/cobalt-containing polypyrrole hydrogel-derived trifunctional electrocatalyst for self-powered overall water splitting. Adv Funct Mater 27:1606497
Shi Y, Zhang J, Bruck AM et al (2017) A tunable 3D nanostructured conductive gel framework electrode for high-performance lithium ion batteries. Adv Mater 29:1603922
Deng Z, Guo Y, Zhao X, Ma PX, Guo B (2018) Multifunctional stimuli-responsive hydrogels with self-healing, high conductivity, and rapid recovery through host–guest interactions. Chem Mater 30:1729–1742
Chen J, Sheng K, Luo P, Li C, Shi G (2012) Graphene hydrogels deposited in nickel foams for high-rate electrochemical capacitors. Adv Mater 24:4569–4573
Mao L, Guan C, Huang X, Ke Q, Zhang Y, Wang J (2016) 3D graphene-nickel hydroxide hydrogel electrode for high-performance supercapacitor. Electrochim Acta 196:653–660
Wang R, Jayakumar A, Xu C, Lee JM (2016) Ni(OH)2 nanoflowers/graphene hydrogels: a new assembly for supercapacitors. ACS Sustain Chem Eng 4:3736–3742
Javadi M, Gu Q, Naficy S et al (2018) Conductive tough hydrogel for bioapplications. Macromol Biosci 18:1700270
De B, Kuila T, Kim NH, Lee JH (2017) Carbon dot stabilized copper sulphide nanoparticles decorated graphene oxide hydrogel for high performance asymmetric supercapacitor. Carbon 122:247–257
Kianfar E (2021) Investigation of the effect of crystallization temperature and time in synthesis of SAPO-34 catalyst for the production of light olefins. Pet Chem 61:527–537. https://doi.org/10.1134/S0965544121050030
Huang X, Zhu Y, Kianfar E (2021) Nano biosensors: properties, applications and electrochemical techniques. J Mater Res Technol 12:1649–1672. https://doi.org/10.1016/j.jmrt.2021.03.048
Kianfar E (2021) Protein nanoparticles in drug delivery: animal protein, plant proteins and protein cages, albumin nanoparticles. J Nanobiotechnol 19:159. https://doi.org/10.1186/s12951-021-00896-3
Kianfar E (2021) Magnetic nanoparticles in targeted drug delivery: a review. J Supercond Novel Magn. https://doi.org/10.1007/s10948-021-05932-9
Syah R, Zahar M, Kianfar E (2021) Nanoreactors: properties, applications and characterization. Int J Chem Reactor Eng 19(10):981–1007. https://doi.org/10.1515/ijcre-2021-0069
Raya I, Kzar HH, Mahmoud ZH, Al Ayub Ahmed A, Ibatova AZ, Kianfar E (2021) A review of gas sensors based on carbon nanomaterial. Carbon Lett. https://doi.org/10.1007/s42823-021-00276-9
Majdi HS, Latipov ZA, Borisov V et al (2021) Nano and battery anode: a review. Nanoscale Res Lett 16:177. https://doi.org/10.1186/s11671-021-03631-x
Bokov D, Turki Jalil A, Chupradit S, Suksatan W, Javed Ansari M, Shewael IH, Valiev GH, Kianfar E (2021) Nanomaterial by sol–gel method: synthesis and application. Adv Mater Sci Eng 2021:21. https://doi.org/10.1155/2021/5102014
Jasim SA, Kadhim MM, Kn V et al (2022) Molecular junctions: introduction and physical foundations, nanoelectrical conductivity and electronic structure and charge transfer in organic molecular junctions. Braz J Phys 52:31. https://doi.org/10.1007/s13538-021-01033-z
Ansari MJ, Kadhim MM, Hussein BA et al (2022) Synthesis and stability of magnetic nanoparticles. BioNanoSci. https://doi.org/10.1007/s12668-022-00947-5
Krishna Sindhu C, Naga Sowmya A, Haveela B, Kavya Nandini G, Shaik S (2021) Design of frequency reconfigurable microstrip antenna. Natl J Antennas Propag 3(1):16–21. https://doi.org/10.31838/NJAP/03.01.04
Sadulla S (2020) A review on finfets from device to circuit. J VLSI Circuits Syst 2(1):11–13. https://doi.org/10.31838/jvcs/02.01.03
Srilakshmi K, Preethi K, Afsha Md, Pooja Sree N, Venu M (2022) Advanced electricity billing system using aurdino uno. Int J Commun Comput Technol 10(1):1–3. https://doi.org/10.31838/ijccts/10.01.01
Enany MA, Farahat MA, Otay MI (2021) Optimal design and operation of fast charging station for electric vehicle via renewable energy in Wadi El-Natrun-El Alamein Road, Egypt. Int J Sustain Energy Environ Res 10(1):38–46. https://doi.org/10.18488/journal.13.2021.101.38.46
Al-Shawi SG, Andreevna Alekhina N, Aravindhan S, Thangavelu L, Elena A, Viktorovna Kartamysheva N, Rafkatovna Zakieva R (2021) Synthesis of NiO nanoparticles and sulfur, and nitrogen co doped-graphene quantum dots/nio nanocomposites for antibacterial application. J Nanostruct 11(1):181–188
Wang H, Zhu B, Jiang W et al (2014) A mechanically and electrically self-healing supercapacitor. Adv Mater 26:3638–3643
Wang K, Zhang X, Li C et al (2015) Chemically crosslinked hydrogel film leads to integrated flexible supercapacitors with superior performance. Adv Mater 27:7451–7457
Huang Y, Zhong M, Shi F et al (2017) An intrinsically stretchable and compressible supercapacitor containing a polyacrylamide hydrogel electrolyte. Angew Chem Int Ed 56:9141–9145
Panchal H, Sadasivuni KK, Ahmed AA, Hishan SS, Doranehgard MH, Essa FA, Shanmugan S, Khalid M (2021) Graphite powder mixed with black paint on the absorber plate of the solar still to enhance yield: an experimental investigation. Desalination 520:115349
Ahmadian E, Maleki S, Sharifi S, Eftekhari A, Samiei M (2020) Hyaluronic acid hydrogel nanoscaffolds: production and assessment of the physicochemical properties. Euras Chem Commun 2(1):51–58. https://doi.org/10.33945/sami/ecc.2020.1.6
Shinde R, Adole VA (2021) Anti-microbial evaluation, experimental and theoretical insights into molecular structure, electronic properties, and chemical reactivity of (E)-2-((1H-indol-3-yl)methylene)-2,3-dihydro-1H-inden-1-one. J Appl Organomet Chem 1(2):48–58. https://doi.org/10.22034/jaoc.2021.278742.1011
Sanjeev R, Jagannadham V, Ravi R (2020) Can non-bonded pair of electrons of Sp3 nitrogen with two single σ-bonds on either side still transmit substituent electronic effects to the reaction site? Reversal of attenuation effect by Sp3 nitrogen—a chemical education perspective. Chem Methodol 4(1):106–114. https://doi.org/10.33945/sami/chemm.2020.1.10
Ozkendir OM, Gunaydin S, Mirzaei M (2019) Electronic structure study of the LiBC3 borocarbide graphene material. Adv J Chem Sect B 1(1):37–41. https://doi.org/10.33945/sami/ajcb.2019.1.7
Chen TC, Rajiman R, Elveny M, Guerrero JW, Lawal AI, Dwijendra NK, Surendar A, Danshina SD, Zhu Y (2021) Engineering of novel Fe-based bulk metallic glasses using a machine learning-based approach. Arab J Sci Eng 46(12):12417–12425. https://doi.org/10.1007/s13369-021-05966-0)
Qaderi J (2020) A brief review on the reaction mechanisms of CO2 hydrogenation into methanol. Int J Innov Res Sci Stud 3(2):33–40. https://doi.org/10.53894/ijirss.v3i2.31
Watandost H, Achak J, Haqmal A (2021) Oxidation of hydrogels based of sodium alginate and MnO2 as catalyst. Int J Innov Res Sci Stud 4(4):191–199. https://doi.org/10.53894/ijirss.v4i4.77
Issa M (2022) Rapid enzymatically reduction of zincum gluconicum for the biomanufacturing of zinc oxide nanoparticles by mycoextracellular filtrate of penicillium digitatum (Pdig-B3) as a soft green technique. Arch Razi Inst 77(1):91–100. https://doi.org/10.22092/ari.2021.356422.1841
Fan FR, Tang W, Wang ZL (2016) Flexible nanogenerators for energy harvesting and self-powered electronics. Adv Mater 28:4283–4305
Hinchet R, Seung W, Kim SW (2015) Recent progress on flexible triboelectric nanogenerators for selfpowered electronics. Chemsuschem 8:2327–2344
Pu X, Liu M, Chen X et al (2017) Ultrastretchable, transparent triboelectric nanogenerator as electronic skin for biomechanical energy harvesting and tactile sensing. Sci Adv 3:e1700015
Bai Y, Jiang Y, Chen B et al (2014) Cyclic performance of viscoelastic dielectric elastomers with solid hydrogel electrodes. Appl Phys Lett 104:062902
Chen B, Bai Y, Xiang F et al (2014) Stretchable and transparent hydrogels as soft conductors for dielectric elastomer actuators. J Polym Sci B 52:1055–1060
Li T, Li G, Liang Y et al (2017) Fast-moving soft electronic fish. Sci Adv 3:e1602045
Larson C, Peele B, Li S et al (2016) Highly stretchable electroluminescent skin for optical signaling and tactile sensing. Science 351:1071–1074
Li S, Peele BN, Larson CM, Zhao H, Shepherd RF (2016) A stretchable multicolor display and touch interface using photopatterning and transfer printing. Adv Mater 28:9770–9775
Yang CH, Chen B, Zhou J, Chen YM, Suo Z (2016) Electroluminescence of giant stretchability. Adv Mater 28:4480–4484
Yang CH, Zhou S, Shian S, Clarke DR, Suo Z (2017) Organic liquid-crystal devices based on ionic conductors. Mater Horiz 4:1102–1109
Kim CC, Lee HH, Oh KH, Sun JY (2016) Highly stretchable, transparent ionic touch panel. Science 353:682–687
Zhou Y, He B, Yan Z, Shang Y, Wang Q, Wang Z (2018) Touch locating and stretch sensing studies of conductive hydrogels with applications to soft robots. Sensors 18:569
Bella F, Galliano S, Falco M et al (2017) Approaching truly sustainable solar cells by the use of water and cellulose derivatives. Green Chem 19:1043–1051
Liu T, Liu M, Dou S et al (2018) Triboelectric-nanogenerator-based soft energy-harvesting skin enabled by toughly bonded elastomer/hydrogel hybrids. ACS Nano 12:2818–2826
Keplinger C, Sun JY, Foo CC, Rothemund P, Whitesides GM, Suo Z (2013) Stretchable, transparent, ionic conductors. Science 341:984–987
Kweon OY, Samanta SK, Won Y, Yoo JH, Oh JH (2019) Stretchable and self-healable conductive hydrogels for wearable multimodal touch sensors with thermoresponsive behavior. ACS Appl Mater Interfaces 11:26134–26143
Sun JY, Keplinger C, Whitesides GM, Suo Z (2014) Ionic skin. Adv Mater 26:7608–7614
Liu S, Zheng R, Chen S et al (2018) A compliant, self-adhesive and self-healing wearable hydrogel as epidermal strain sensor. J Mater Chem C 6:4183–4190
Morelle XP, Illeperuma WR, Tian K, Bai R, Suo Z, Vlassak JJ (2018) Highly stretchable and tough hydrogels below water freezing temperature. Adv Mater 30:1801541
Lei Z, Wang Q, Sun S, Zhu W, Wu P (2017) A bioinspired mineral hydrogel as a self-healable, mechanically adaptable ionic skin for highly sensitive pressure sensing. Adv Mater 29:1700321
Si Y, Wang L, Wang X, Tang N, Yu J, Ding B (2017) Ultrahigh-water-content, superelastic, and shape-memory nanofiber-assembled hydrogels exhibiting pressure-responsive conductivity. Adv Mater 29:1700339
Liu YJ, Cao WT, Ma MG, Wan P (2017) Ultrasensitive wearable soft strain sensors of conductive, self-healing, and elastic hydrogels with synergistic “soft and hard” hybrid networks. ACS Appl Mater Interfaces 9:25559–25570
Deng Y, Hussain I, Kang M et al (2018) Self-recoverable and mechanical-reinforced hydrogel based on hydrophobic interaction with self-healable and conductive properties. Chem Eng J 353:900–910
Liu S, Li K, Hussain I et al (2018) A conductive self-healing double network hydrogel with toughness and force sensitivity. Chem A Eur J 24:6632–6638
Liu S, Oderinde O, Hussain I, Yao F, Fu G (2018) Dual ionic cross-linked double network hydrogel with self-healing, conductive, and force sensitive properties. Polymer 144:111–120
Shao C, Wang M, Meng L et al (2018) Mussel-inspired cellulose nanocomposite tough hydrogels with synergistic self-healing, adhesive, and strain-sensitive properties. Chem Mater 30:3110–3121
Tran VT, Mredha MTI, Pathak SK, Yoon H, Cui J, Jeon I (2019) Conductive tough hydrogels with a staggered ion-coordinating structure for high self-recovery rate. ACS Appl Mater Interfaces 11:24598–24608
Lai J, Zhou H, Wang M et al (2018) Recyclable, stretchable and conductive double network hydrogels towards flexible strain sensors. J Mater Chem C 6:13316–13324
Schroeder TBH, Guha A, Lamoureux A et al (2017) An electric-eel-inspired soft power source from stacked hydrogels. Nature 552:214–218
Jing X, Mi HY, Lin YJ, Enriquez E, Peng XF, Turng LS (2018) Highly stretchable and biocompatible strain sensors based on mussel-inspired super-adhesive self-healing hydrogels for human motion monitoring. ACS Appl Mater Interfaces 10:20897–20909
Wu J, Han S, Yang T et al (2018) Highly stretchable and transparent thermistor based on self-healing double network hydrogel. ACS Appl Mater Interfaces 10:19097–19105
Wu J, Wu Z, Han S et al (2019) Extremely deformable, transparent, and high-performance gas sensor based on ionic conductive hydrogel. ACS Appl Mater Interfaces 11:2364–2373
Bai Y, Chen B, Xiang F, Zhou J, Wang H, Suo Z (2014) Transparent hydrogel with enhanced water retention capacity by introducing highly hydratable salt. Appl Phys Lett 105:151903
Huo Z, Tao L, Wang S et al (2015) A novel polysulfide hydrogel electrolyte based on low molecular mass organogelator for quasi-solid-state quantum dot-sensitized solar cells. J Power Sources 284:582–587
Liu X, Wu D, Wang H, Wang Q (2014) Self-recovering tough gel electrolyte with adjustable supercapacitor performance. Adv Mater 26:4370–4375
He X, Zhang C, Wang M, Zhang Y, Liu L, Yang W (2017) An electrically and mechanically autonomic self-healing hybrid hydrogel with tough and thermoplastic properties. ACS Appl Mater Interfaces 9:11134–11143
Hao GP, Hippauf F, Oschatz M et al (2014) Stretchable and semitransparent conductive hybrid hydrogels for flexible supercapacitors. ACS Nano 8:7138–7146
Ding H, Zhong M, Kim YJ et al (2014) Biologically derived soft conducting hydrogels using heparin-doped polymer networks. ACS Nano 8:4348–4357
Liao M, Wan P, Wen J et al (2017) Wearable, healable, and adhesive epidermal sensors assembled from mussel-inspired conductive hybrid hydrogel framework. Adv Funct Mater 27:1703852
Hutapea S, Ghazi Al-Shawi S, Chen TC, You X, Bokov D, Abdelbasset WK, Suksatan W (2021). Study on food preservation materials based on nano-particle reagents. Food Sci Technol
Yin G, Alazzawi FJ, Mironov S, Reegu F, El-Shafay AS, Rahman ML, Su CH, Lu YZ, Nguyen HC (2022) Machine learning method for simulation of adsorption separation: comparisons of model’s performance in predicting equilibrium concentrations. Arab J Chem 15(3):103612
Bashar Mahmood H, Obead W, Al-Arubaye N (2022) Anatomical assessments for grey mongoose tongue by scanning electron microscope (Herpestes edwardsii) in Iraq. Arch f Razi Inst 77(1):118–122. https://doi.org/10.22092/ari.2021.356480.1851
Acknowledgements
Department of Chemical Engineering, Arak Branch, Islamic Azad University, Arak, Iran. Young Researchers and Elite Club, Gachsaran Branch, Islamic Azad University, Gachsaran, Iran.
Funding
There is no funding to report for this submission.
Author information
Authors and Affiliations
Contributions
Therefore, on this basis NDT, DTNH, MJCO, and HAL contributed to investigation, concept and design, experimental studies, writing—original draft, and reviewing and editing, and AMA, DOB, KS, HVTM, ATH, and EK were involved in investigation, concept and design, and data curation. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
I wrote to you in regard to your question about naming some people in my article, I must point out that in some cases, help was sought from people and it was necessary to mention the names of these people in order to maintain professional ethics in research issues.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1186/s11671-022-03746-9"
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Trung, N.D., Huy, D.T.N., Jade Catalan Opulencia, M. et al. RETRACTED ARTICLE: Conductive Gels: Properties and Applications of Nanoelectronics. Nanoscale Res Lett 17, 50 (2022). https://doi.org/10.1186/s11671-022-03687-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11671-022-03687-3